Statement H3PO3 is a dibasic acid and its salt Na2PO3 does not exist.
Explanation Being dibasic nature, only two H are replaceable.
A
S is correct but E is wrong.
B
S is wrong but E is wrong.
C
Both S and E are correct and E is correct explanation of S.
D
Both S and E are correct but E is not correct explanation of S.
Video Solution
Text Solution
The correct Answer is:C
HO−H∣P∣∣O−OH , thus only Na2HPO3 and NaH2PO3 salts are possible.
|
Similar Questions
Knowledge Check
H3PO3 is ..... acid
AMonobasicBDibasicCTribasicDNoneStatement H3BO3 is monobasic Lewis acid but its salt Na3BO3 exist.
Explanation H3BO3 reacts with NaOH to give Na3BO3.AS is correct but E is wrong.BS is wrong but E is wrong.CBoth S and E are correct and E is correct explanation of S.DBoth S and E are correct but E is not correct explanation of S.Statement -1 : H3PO2 is a weak monobasic acid and is also strong reducing in nature.
Statement -2 :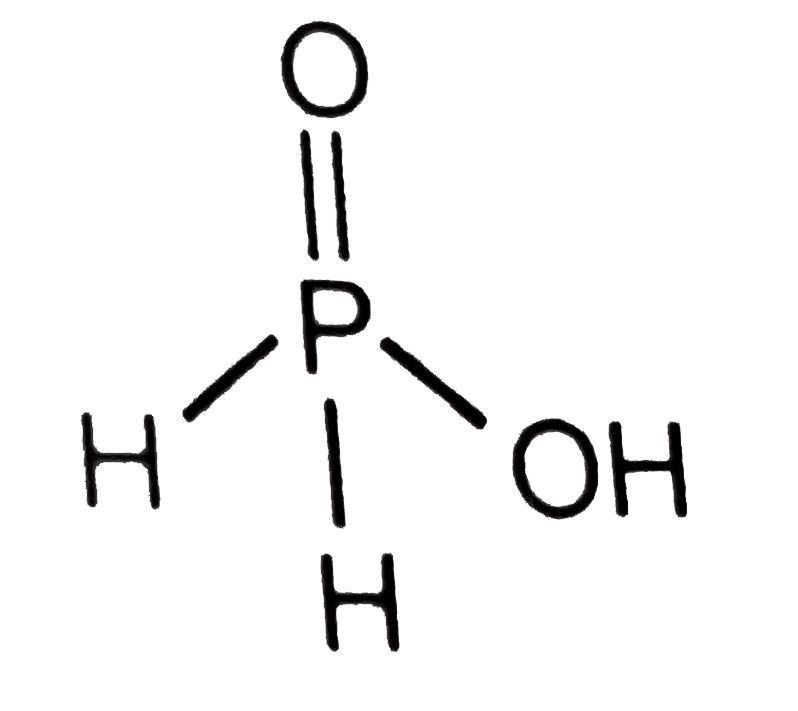 AStatement -1 is true , Statement-2 is true, Statement-2 is a correct explantion for Statement -1.BStatement -1 is true , Statement-2 is true, Statement-2 is a NOT a correct explanation for Statement -1.CStatement -1 is true, Statement- 2 is false.DStatement -1 and Statement- 2 both are False.
AStatement -1 is true , Statement-2 is true, Statement-2 is a correct explantion for Statement -1.BStatement -1 is true , Statement-2 is true, Statement-2 is a NOT a correct explanation for Statement -1.CStatement -1 is true, Statement- 2 is false.DStatement -1 and Statement- 2 both are False.
Why does H3PO3 act as a reducing agent but H3PO4 does not ?
View SolutionWhy does H3PO3 act as a reducing agent but H3PO4 does not ?
View SolutionH3PO3 undergoes disproportionation reaction but H3PO4 does not ? Explain.
View SolutionH3PO2andH3PO3 act as good reducing agents but H3PO4 does not. Explain.
View SolutionH3PO3 is diprotic (or dibasic). Why ?
View Solution

