Some times a reacant undergoes chemical/radioactive changes following two or more different paths to yield two or more different produces respectively. Such reactions are called parallel path reactions. If K1 and K2 are rate constans for the reaction of A follwing two parallel paths, then
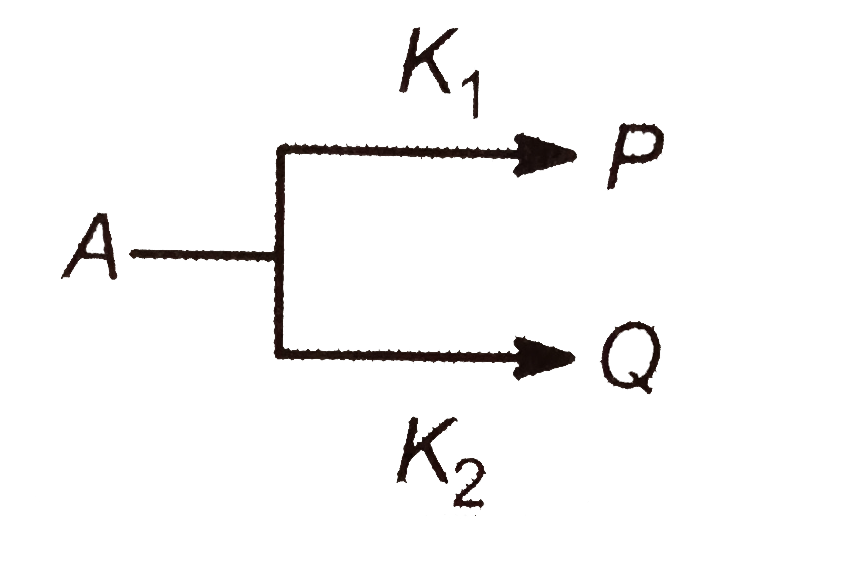
Then Kav=K1+K2
If average life of A for p is T1 and for Q is T2 then:
Tav=T1+T2
Tav=T1T2T1+T2
Tav=T1+T2T1T2
Tav=K1T1+K2T2
The correct Answer is:b
Kav=1Tav
Topper's Solved these Questions
- RADIO ACTIVITYBOOK - P BAHADURCHAPTER - RADIO ACTIVITYEXERCISE - Exercies 8
16 Videos
- RADIO ACTIVITYBOOK - P BAHADURCHAPTER - RADIO ACTIVITYEXERCISE - Exercies 9
99 Videos
- RADIO ACTIVITYBOOK - P BAHADURCHAPTER - RADIO ACTIVITYEXERCISE - Exercies 6
14 Videos
- MOLE AND EQUIVALENT CONCEPTBOOK - P BAHADURCHAPTER - MOLE AND EQUIVALENT CONCEPTEXERCISE - Exercise 9 Advanced numerical problems
61 Videos
- REDOX REACTIONSBOOK - P BAHADURCHAPTER - REDOX REACTIONSEXERCISE - Exercise(8) Statement:Explanation type problems
19 Videos
Similar Questions
Knowledge Check
Some times a reacant undergoes chemical/radioactive changes following two or more different paths to yield two or more different produces respectively. Such reactions are called parallel path reactions. If K1 and K2 are rate constans for the reaction of A follwing two parallel paths, then
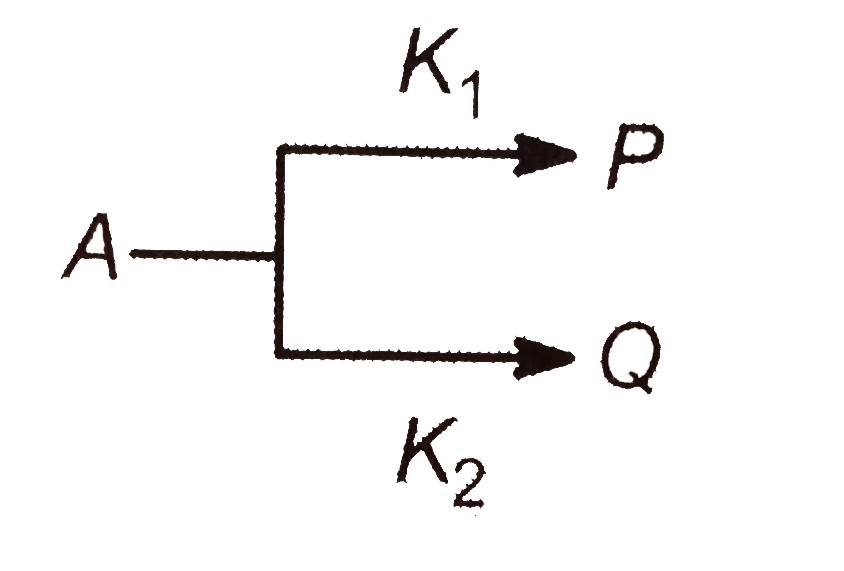
Then Kav=K1+K2
For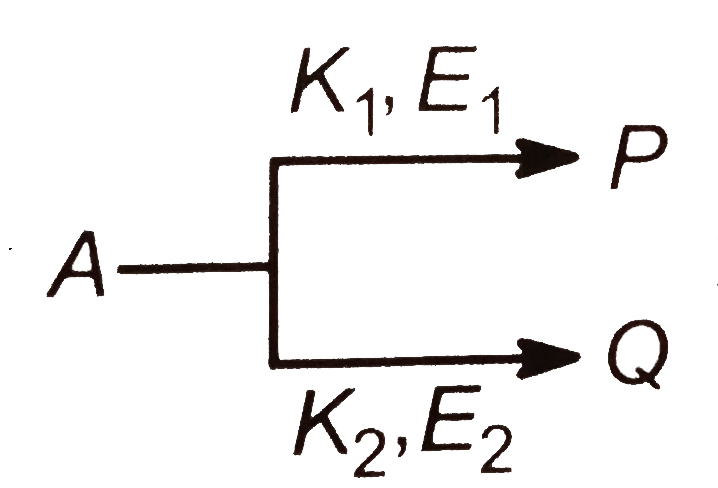 if E1 and E2 are energy fo activations, thenAETotal=E1+E2BETotal=E1−E2CETotal=K1E1+K2E2DETotal=K1E1+K2E2K1+K2
if E1 and E2 are energy fo activations, thenAETotal=E1+E2BETotal=E1−E2CETotal=K1E1+K2E2DETotal=K1E1+K2E2K1+K2Some times a reacant undergoes chemical/radioactive changes following two or more different paths to yield two or more different produces respectively. Such reactions are called parallel path reactions. If K1 and K2 are rate constans for the reaction of A follwing two parallel paths, then
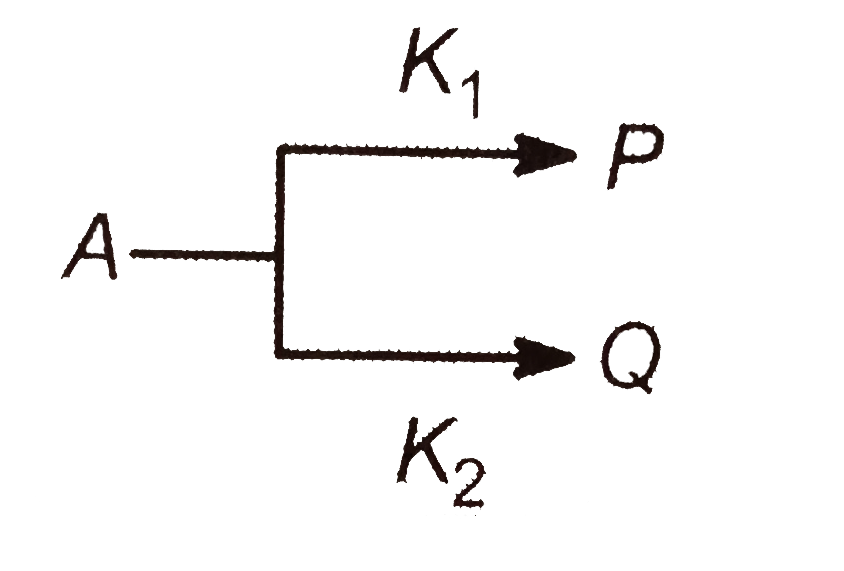
Then Kav=K1+K2
Which one of the following is correct for the above reaction:AFractionl yield of p=K1K2BFractional yield of p=K1+K2KavCFractional yeild of p=K1KavDFractional yield of p=K2KavK1 and K2 are the rate constants of forward and backward reactions. The equilibrium constant K of the reaction is -
AK1×K2BK1−K2CK1K2)DK1+K2K1−K2
A slab consists of two parallel layers of two different materials of same thickness having thermal conductivities K1andK2. The equivalent conductivity of the combination is
View SolutionActivation energy (Ea) and rate constant (K1 and (K2) for a chemical reaction at two different temperatures T1 and T2 are related by:
View SolutionActivation energy (Ea) and rate constants (k1 and k2) of a chemical reaction at two different temperatures (T1 and T2) are related by
View SolutionActivation energy (Ea) and rate constants (k1 and k2) of a chemical reaction at two different temperatures (T1 and T2) are related by
View SolutionActivation energy (Ea) and rate constants (k1 and k2) of a chemical reaction at two different temperatures (T1 and T2) are related by
View Solution

