The crystal structure adopted by iron is shown below. The distance between the nearest iron atoms is :
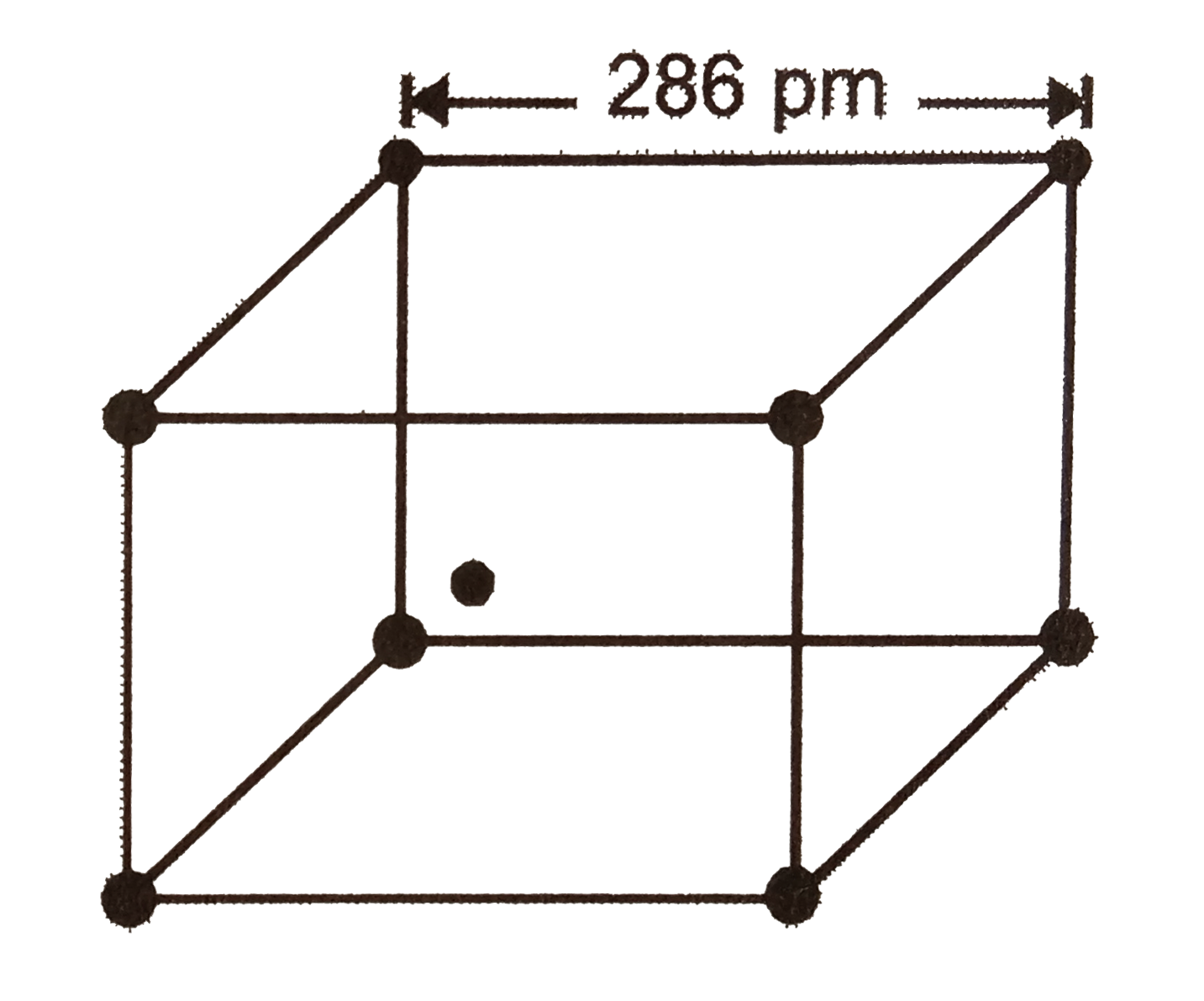
286pm
124pm
143pm
247.6pm
The correct Answer is:d
The nearest neighbours touch along body diagonal.
So, nearest distance =√32×286=247.68pm.
Similar Questions
Calculate the density of silver which crystallises in face-centred cubic from. The distance between nearest metal atoms is 287 pm (Molar mass of Ag = 107.87gmol−1,(N0=6.022×1023mol−1).
View SolutionCalculate the density of silver which crystallises in face-centred cubic from. The distance between nearest metal atoms is 287 pm (Molar mass of Ag = 107.87gmol−1,(N0=6.022×1023mol−1).
View Solution
Knowledge Check
Assertiion : X - rays are used for studying the structure of crystals.
Reason : The distance between the atoms of crystals is of the order of wavelength of X - rays.AIf both assertion and reason are true and reason is the correct explanation of assertion.BIf both assertion and reason are true but reason is not the correct explanation of assertion.CIf assertion is true but reason is false.DIf assertion and reason both are false.The structure of iron pentacarbonyl is:
A(a) square planarB(b) triangularC(c) trigonal bipyramidalD(d) none of theseAB crystal has CsCl type structure. If edge length of unit cell is 100 pm then nearest distance between cation and anion is :-
A50 pmB100 pmC173.2 pmD86.6 pm
What type of crystal structure is shown by graphite ?
View SolutionThe arrangement in an ideal crystal AB and a defect structured crystal is shown below.
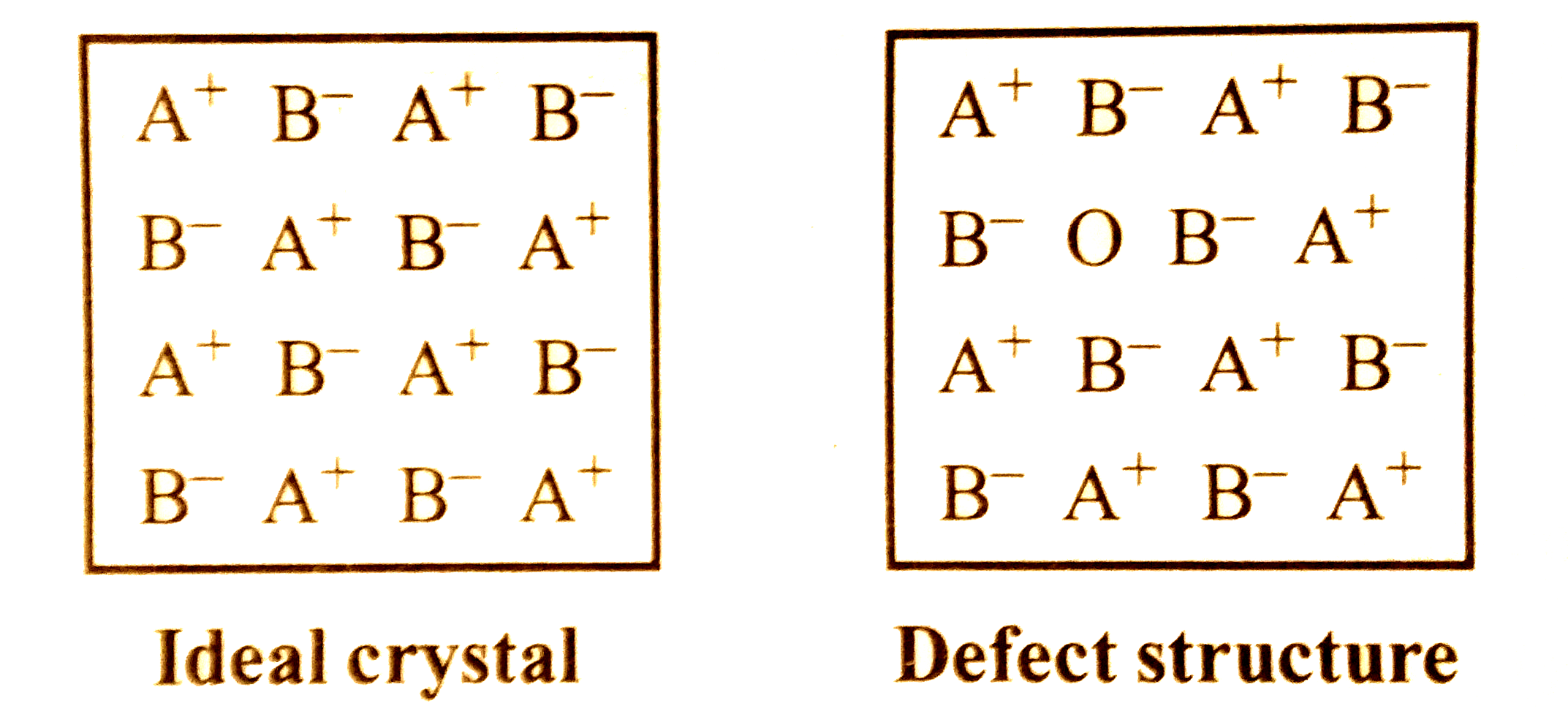
The illustrates the example of
View SolutionIron crystallizes in several forms. At 1185 K , the body centred cubic α- form of iron changes to the face centred cubic γ- form of iron. Assuming that the distance betweem the nearest neighbours is the same in two forms at the transition temperature, calcuate the ratio of the density of α- form to that of γ- form at the transition temperature.
View SolutionPlatinum crystallize in a face-centred cubic crystal with a unite cell length a. the distance between nearest neighbours is:
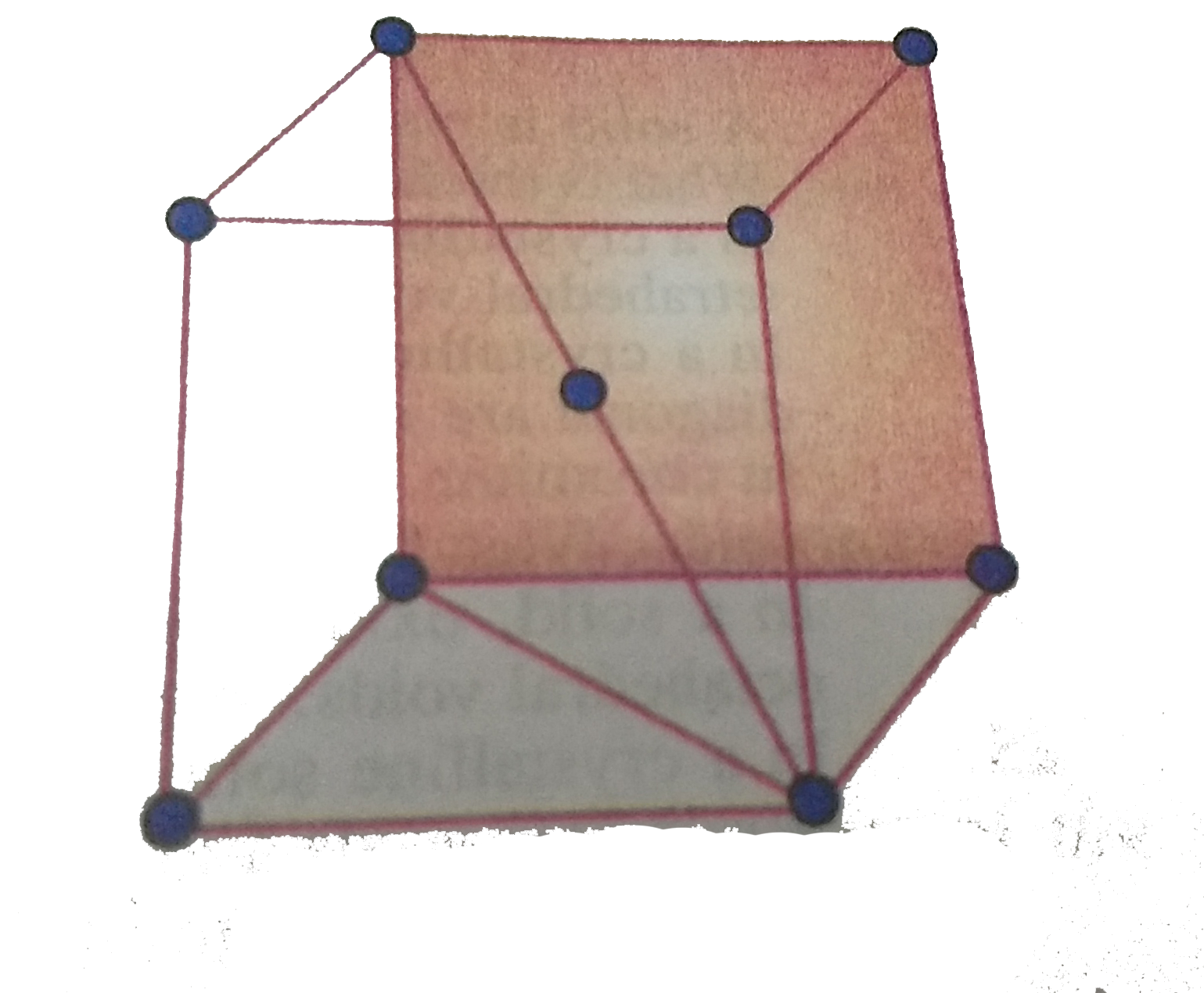
View SolutionPotassium crystallises in a bcc lattice as shown in figure :
(a) What is the distance the nearest neighbours ?
(b) What is the distance between the next nearest neighbours ?
(c) How many nearest neighbours does each potassium atom have ?
(d) How many next nearest neighbours does each potassium atom have ?
View Solution

