Period 2 elements
Boron, carbon, nitrogen, oxygen
Topper's Solved these Questions
- PERIODIC CLASSIFICATION OF ELEMENTS BOOK - TARGET PUBLICATIONCHAPTER - PERIODIC CLASSIFICATION OF ELEMENTS EXERCISE - Give reasons
5 Videos
- PERIODIC CLASSIFICATION OF ELEMENTS BOOK - TARGET PUBLICATIONCHAPTER - PERIODIC CLASSIFICATION OF ELEMENTS EXERCISE - Distinguish between
2 Videos
- PERIODIC CLASSIFICATION OF ELEMENTS BOOK - TARGET PUBLICATIONCHAPTER - PERIODIC CLASSIFICATION OF ELEMENTS EXERCISE - Match the following
4 Videos
- METALLURGYBOOK - TARGET PUBLICATIONCHAPTER - METALLURGYEXERCISE - CHAPTER ASSESSMENT
19 Videos
Similar Questions
The third period contains only eight elements even though the electron capacity of the third shell is 18.
View SolutionWhat are the basic differences between Mendeleev's periodic table and modern periodic table?
View SolutionRearrange the columns 2 and 3 so as to match with the column 1.
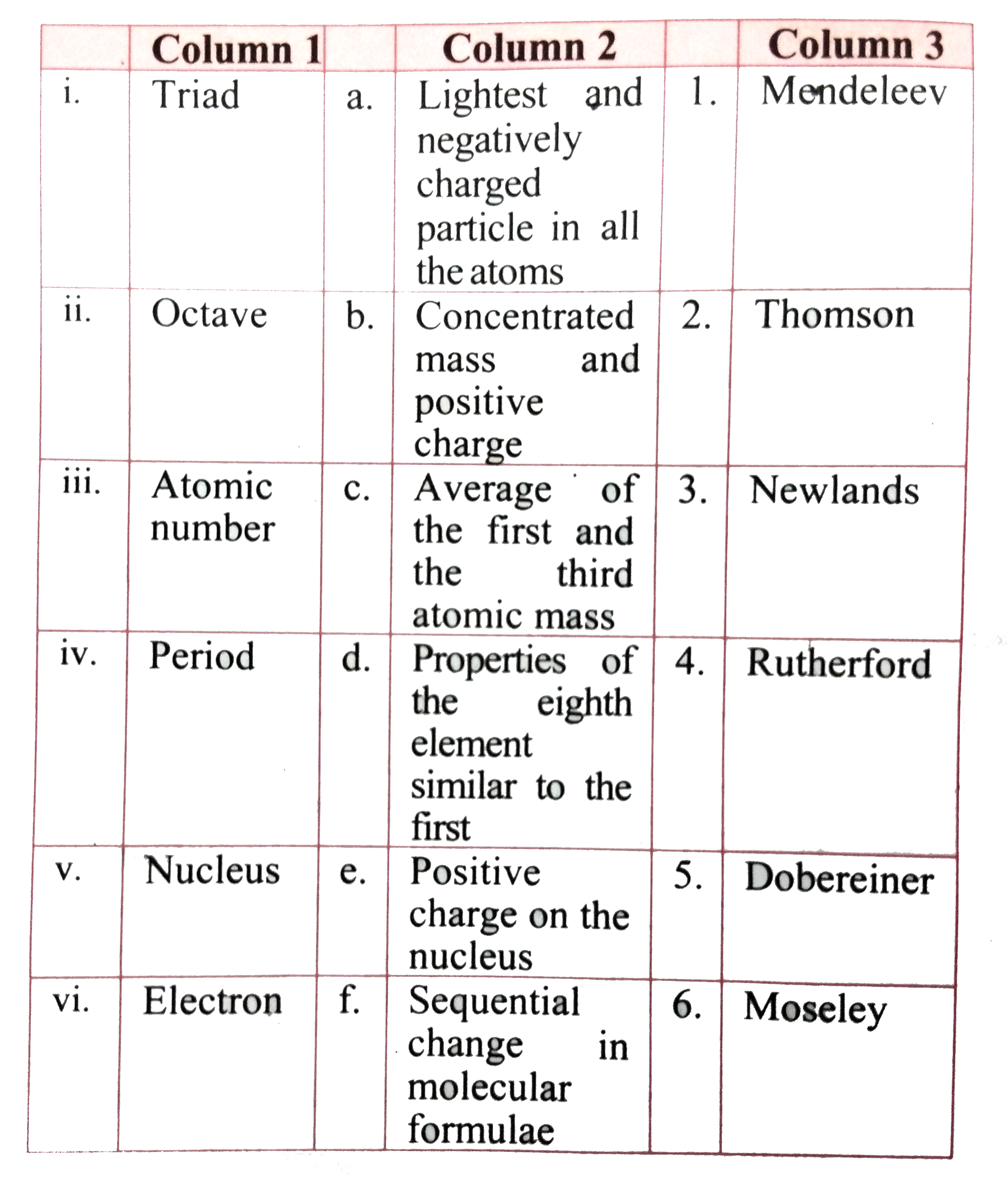
View SolutionWhich one of the following metals does catch fire rapidly when it comes in contact with air?
View Solutionआवर्त सारणी में कौन-से आवर्त के तत्व प्रारूपी तत्व है? उनके नाम लिखिए।
View SolutionThree nonmetallic elements in the period 2.
View SolutionConsider the elements of period 2 in the modern periodic table. Answer the following questions with explanation. Which is the most electropositive element in this period?
View Solutionआवर्त सारणी के वर्ग - 2 के तत्त्व वर्ग -1 के तत्त्वों के अपेक्षा होते है
View Solutionआवर्त सारणी के वर्ग -2 के तत्त्व वर्ग -1 के तत्त्वों की अपेक्षा होते है
View SolutionNumber of elements present in period - 2 of the long form periodic table _________
View SolutionPeriod 2 consists of eight elements.
View SolutionElements of a group and Elements of a period
View SolutionThe electronic configuration of an element is 2, 8, 3, then it is an element of ............ period.
View SolutionThird period contain 8 elements. Fourth period contain 18 elements. Seven period contain 32 elements.
View SolutionDraw the electronic configuration of the period 2 element of first group in the periodic table
View Solution

