Write the balanced equations for the following reactions :
* (i) H2S2O7(1)+H2O(1)→H2SO4(1)
Video Solution
Text Solution
Step 1 : Rewrite the given equation as it is H2S2O7(1)+H2O(1)→H2SO4(1)
Step 2 : Write the number of atoms of each element in the unbalanced equation on both sides of equations.

Step 3 : To equalise the nuber of hydrogen atoms , sulphur atoms and oxygen atoms we use 2 the coefficient or factor in the product.
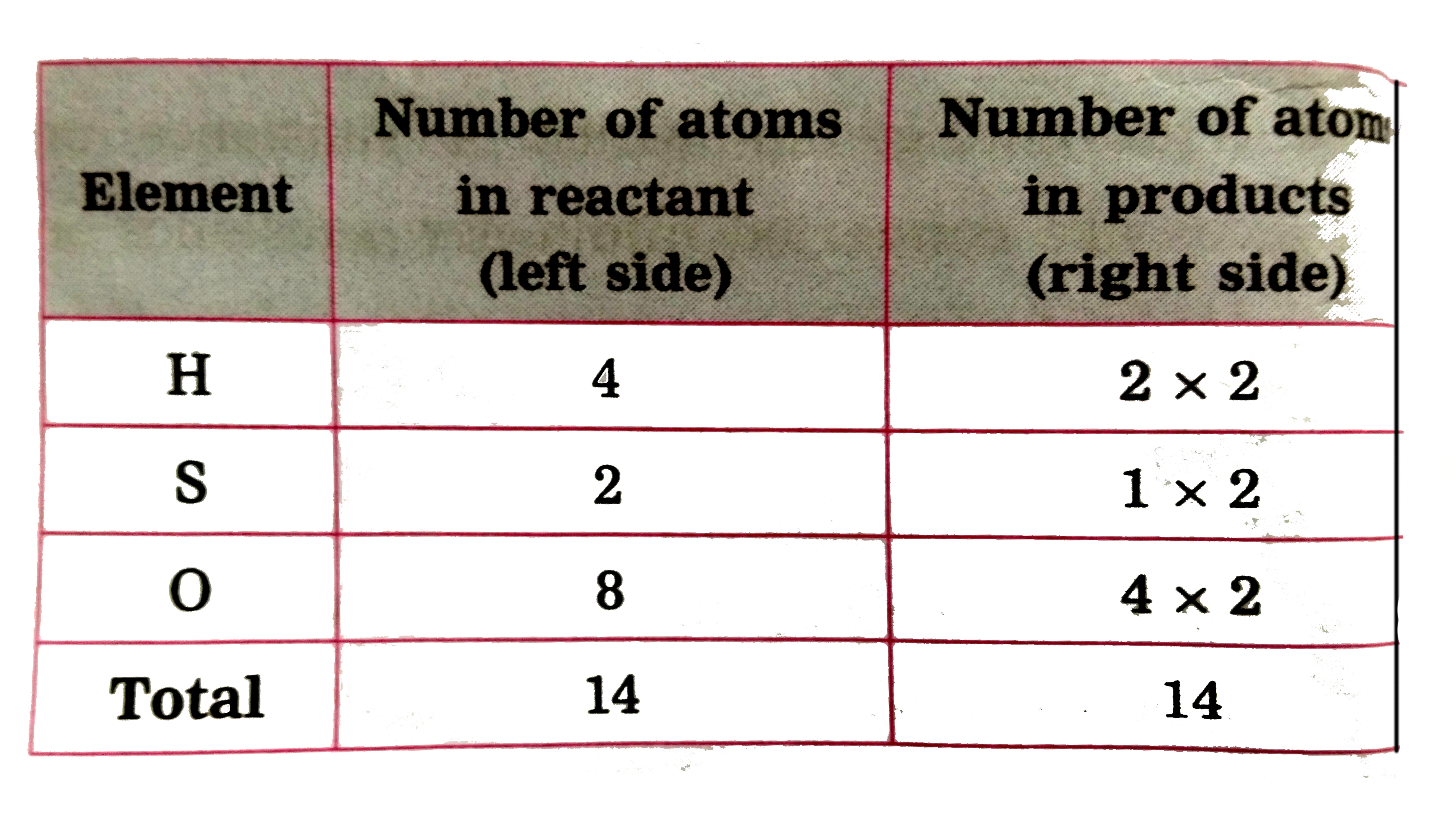
Now the equation becomes H2S2O7+H2O→2H2SO4
Now , count the atoms of each element on both sides of the equation. The number of atoms on both sides are equal. Hence , the balanced equation is
H2S2O7+H2O→2H2SO4
Now indicate physical states of the reactants and products.
H2S2O7(1)+H2O(1)→2H2SO4(1)
|
Similar Questions
H_(2)underline(S_(2))O_(8)+H_(2)O to H_(2)SO_(4)+H_(2)O_(2)
View SolutionHow many of the following molecules do not contain peroxide linkage?
H2S2O5,H2SO5,H2S2O8,H4P2O8,H3PO5,H2S2O6
View SolutionHypothetical scheme is given below for one molecule of acids.
H2O2SO3−−→H2SO5SO3−−→(X)↓Peroxomonosulphuric acidH2O2SO2−−→H2S2O3SO3−−→(Y)↓Thiosulphuric acidH2OnSO3−−→H2S(n+1)O3SO3−−→(Z)
Find the sum of peroxylinkage in X,Y and Z.
View SolutionBalance the following chemical equations.
c) H2+O2→H2O
View SolutionH2SO3,H2S2O4व H2S2O7 की संरचनायें बनाइये।
View Solutionनिम्नलिखित रासायनिक समीकरणों को संतुलित कीजिए।
H2S+O2→SO2+H2O
View SolutionComplete and balance the following equations:
(i) FeSO4+H2O2+H2SO4→………+…………….
(ii) H2O2+Ag2O→………+…………….
View SolutionH_(2)underline(S_(2))O_(8)+H_(2)O to H_(2)SO_(4)+H_(2)O_(2)
View Solution