Complete the following reactions :
(1) Zn(s)+H2O(g)→……..
(2) C(s)+O2(g)Complete−−−−−−→Combustion……
(1) Zn(s)+H2O(g)→ZnO(s)+H2(g)
(2) C(s)+O2(g)Complete−−−−−−→CombustionCO2(g) (acidic oxide)
Similar Questions
Knowledge Check
Complete the following reactions,
(i) H2(g)+MnO(s)Δ−−→Mn(s)+H2O(g)
(ii) CO(g)+H2(g)Δ−−−−→Catalyst
(iii) C3H8(g)+3H2O(g)Δ−−−−→Catalyst
(iv) Zn(s)+NaOH(aq)Heat−−→
After completing the above reactions in the balanced equations the coefficient for H2(g) may beA1,2,7,1B1,2,4,2C1,2,1,1D1,3,4,2
Complete the following equations
(i) PbS(s)+H2O2(aq)→
(ii)CO(g)+2H2(g)cobalts−−−−→catalyst
View SolutionComplete the following equations
(i) PbS(s)+H2O2(aq)→
(ii)CO(g)+2H2(g)cobalt−−−−→catalyst
View SolutionComplete the following reactions :
(1) N2(g)+3H2(g)Proper tem.−−−−−−−→high pressure…..
(2) 2C(s)+O2(g)Partial combustion−−−−−−−−−−→……
View SolutionComplete the following reactions:
a.H2(g)+MmOo(s)Δ−−→
b. CO(g)+H2(g)Δ−−−−→catalyst
c. C3H8(g)+3H2O(g)Δ−−−−→catalyst
d. Zn(s)+NaOH(aq)Heat−−→
View SolutionComplete the following reactions:
a.H2(g)+MmOo(s)Δ−−→
b. CO(g)+H2(g)Δ−−−−→catalyst
c. C3H8(g)+3H2O(g)Δ−−−−→catalyst
d. Zn(s)+NaOH(aq)Heat−−→
View SolutionComplete the following reaction:
o−HOOC(A)−C6H4−CH2−PhSOCl2−−−→(B)
Anhyd−−−−−→AlCl3(C)Zn+Hg−−−−−−−→+HCl(D)SorSe−−−−−→600∘C(E)
View SolutionComplete the following reactions :
(a) C_6H_6 + Ac_2 O overset(AlCl_3)rarr (A) overset(1.Br_2//overset(Ө)O H) underset(2.H_3 O^(oplus))rarr (B)
(b)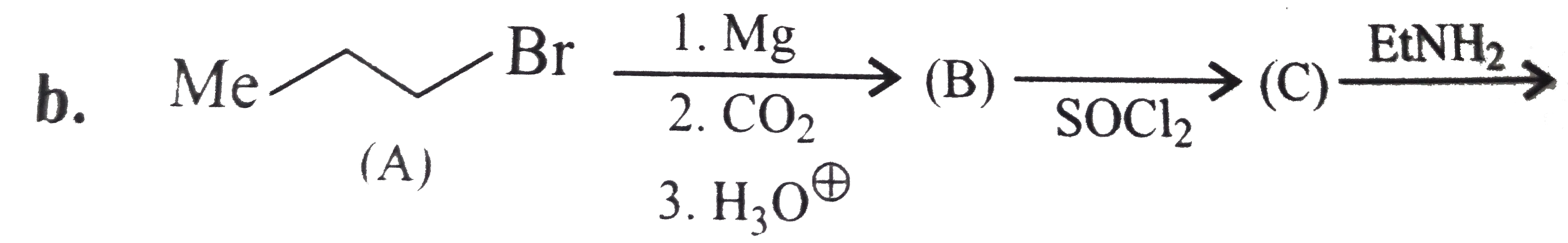
( c)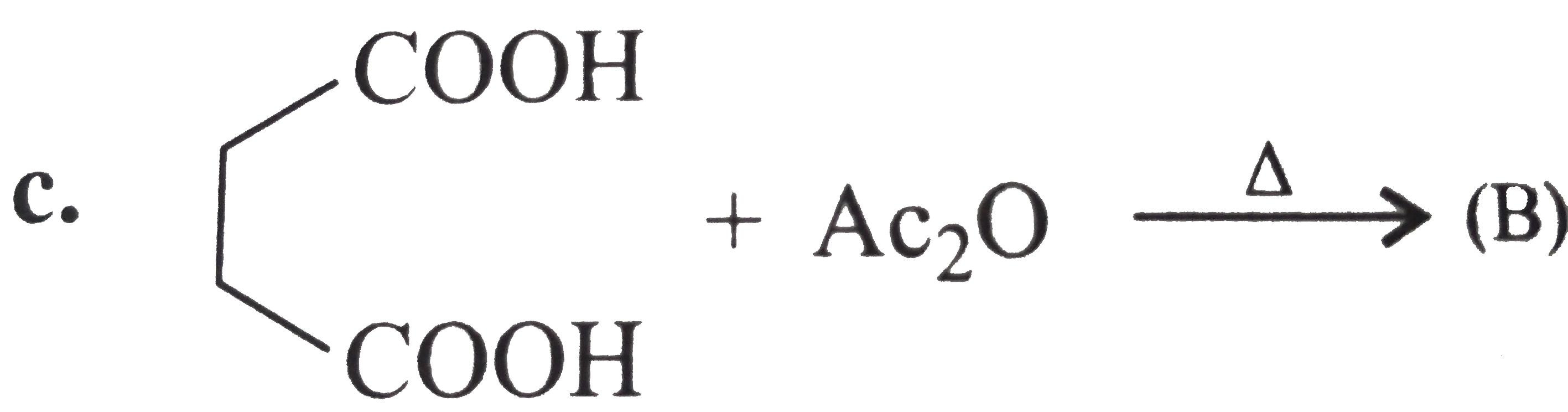
(d)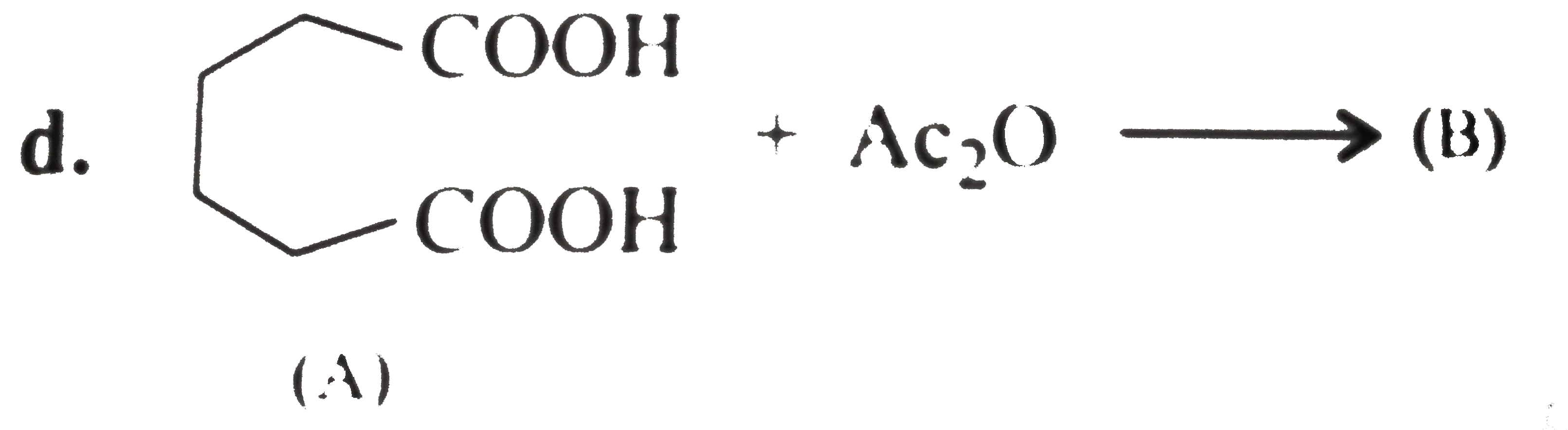
( e)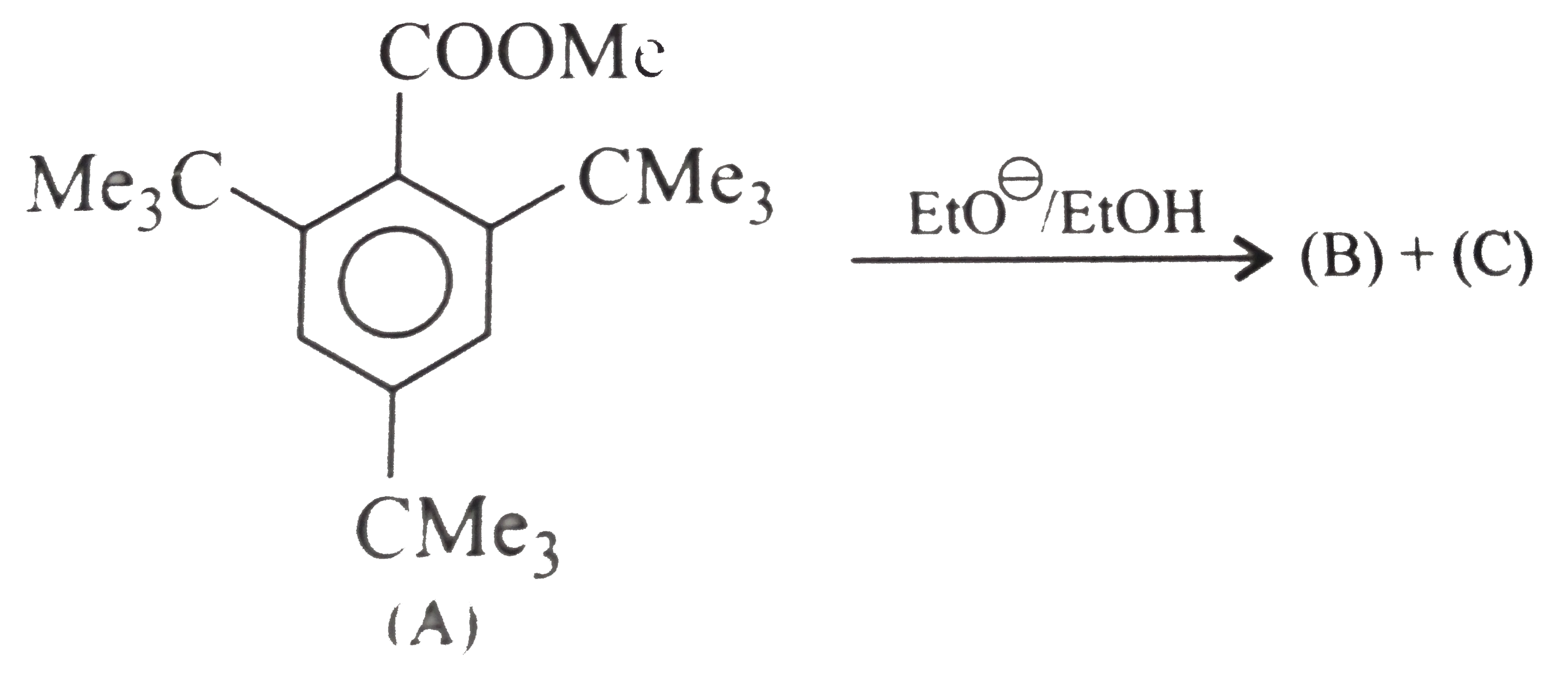
(f)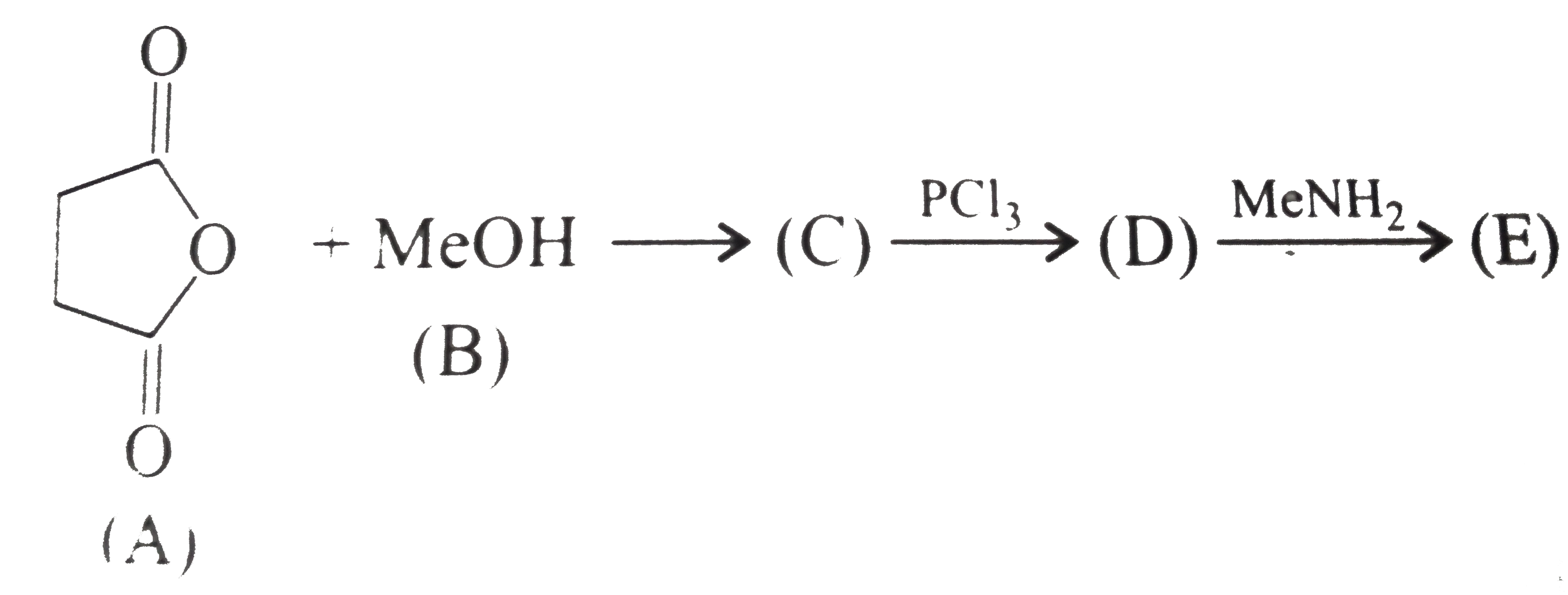
(g)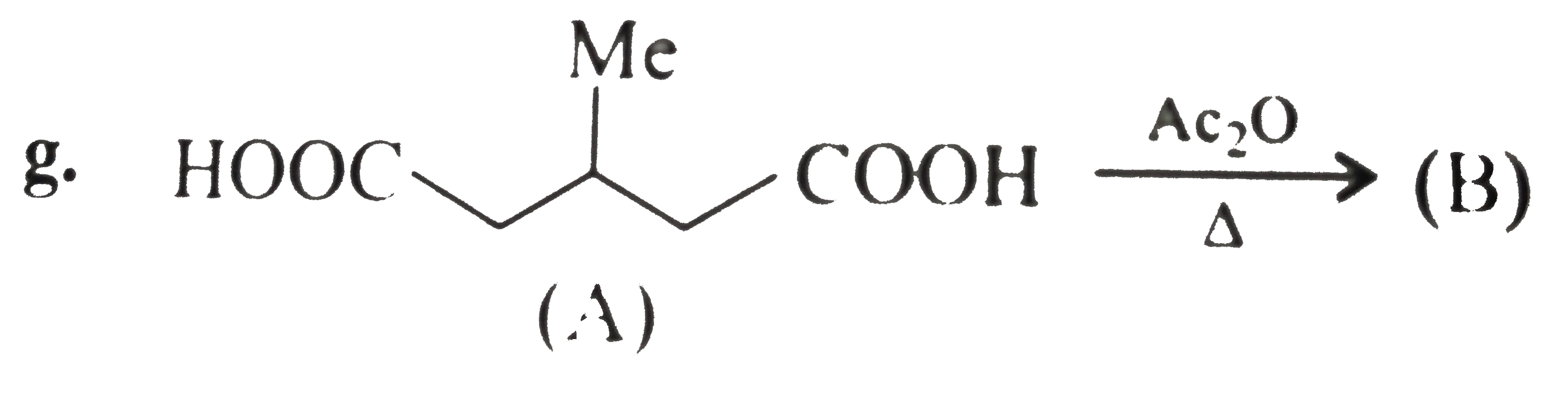 .
.
View Solution