A beam of 35.0keV electrons strikes a molybdenum target, generating the X-rays. What is the cutoff wavelength ?
35.5±
40.0±
15.95±
18.2±
The correct Answer is:A
The cut-off wavelength λmin corresponds to an electron transferring (approximately) all of its energy to an X−ray photon, thus producting a photon with the greatest possible frequency and least possible wavelength.
From relation
λmin=hcK0=(4.14×10−5)(3×108)35.0×103
=3.55×10−11=35.5±.
Similar Questions
A beam of 35.0 keV electrons strikes a molybdenum target, generating the x rays whose spectrum is shown in Fig. What is the cutoff wavelength?
View SolutionThe x rays shown are produced when 35.0 ke V electrons strike a molybdenum (Z=42) target. If the accelerating potential is maintained at this value but a silver (Z=47) target is used instead, what values of (a) λmin, (b) the wavelength of the Kα line, and (c ) the wavelength of the Kβ line result? The K, L, and M atomic x-ray levels for silver are 25.51, 3.56, and 0.53 keV.
View Solution
Knowledge Check
A beam of 350 keV electrons a molybdenum target, generating the X-rays. What is the cut-off wavelength?
A3.55 pmB400 pmC1595 pmD182 pmA beam of electrons having energy 28 keV each strikes a target generating X rays. The minimum wave length λmin (called cut off wavelength ) of the X rays generated is
A4.4 nmB44 nmC0.044 nmD0.44 nmIn producing X-rays a beam of electrons accelerated by a potential difference V is made to strike a metal target. For what value of V,X-rays will have the lowest wavelength of 0.3094 Å
A10 kVB20 kVC30 kVD40 kV
A beam of electrons having energy 28 keV each strikes a target generating X rays. The minimum wave length λmin (called cut off wavelength ) of the X rays generated is
View Solution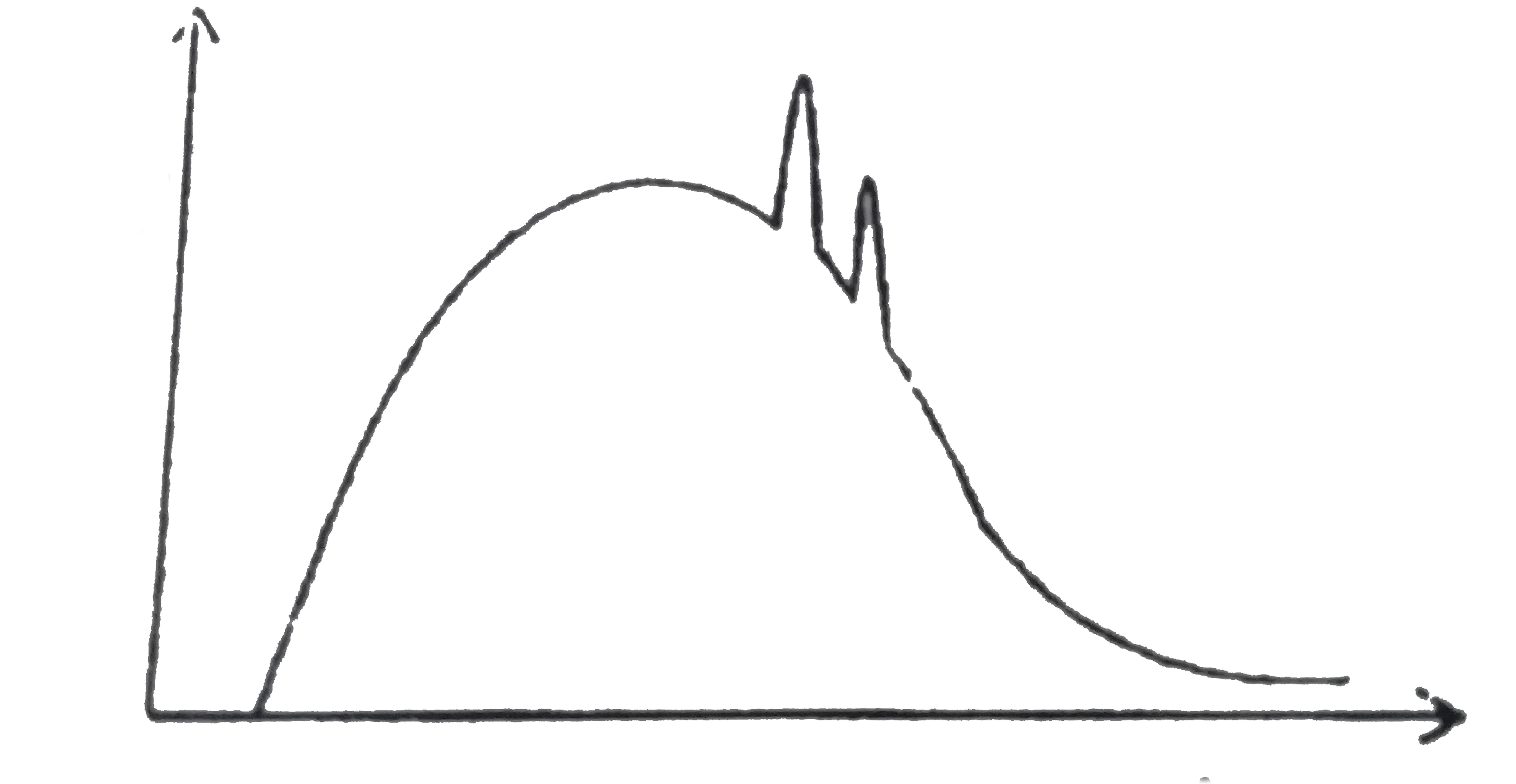
A beam of electrons striking a cooper target produces X-rays. Its spectrum is as shown. Keeping the voltage same if the copper target is replaced with a different metal, the cut-off wavelength and characteristic lines of the new spectrum will change in comparison with old as:
View SolutionIn an x-ray tube, electrons with energy 35 keV are incident on a cobalt (Z=27) target. Determine the cutoff wavelength for x-ray production.
View SolutionElectrons with de-Broglie wavelength λ fall on the target in an X-ray tube. The cut-off wavelength of the emitted X-ray is
View SolutionThe kinetic energy of electrons that strike the target is increased , then the cut-off wavelength of continuous X-rays spectrum
View Solution

