Given below are the sets of quantum nummbers for given orbital. Name these orbitals .
(a) n=3l=1m=−1
(b) n=5l=2m=0
(c ) n=4l=1m=±1
(d) n=2l=0m=0
(e). n=4l=2m=±1
(a) ∴N=3and l=1∴3p
Also m=−1∴3px or 3py
(b)5dz2
(c)4px or 4py
(d) 2s
(e)4dx2−y2or4d⋅xy.
Topper's Solved these Questions
Similar Questions
Given below are sets of quantum numbers for given orbitals. Name these orbitals.
(a) n=3,l=1 (b) n=5,l=2 (c) n=4,l=1 (d) n=2,l=0 (e) n=4,l=2
View SolutionGiven below are tehsets of quantum numbers for given orbitals. Name these orbitals.
a. n=2,l=1,ml=1 b. n=4,l=2,ml=0
c.n=3,l=1,ml=±1 d. n=3,l=2,ml=±2
View Solution
Knowledge Check
If a∫0f(2a−x)dx=manda∫0f(x)dx=n, then 2a∫0f(x)dx is equal to
A2m+nBm+2nCm−nDm+n⎧⎪ ⎪⎨⎪ ⎪⎩NH(CH2)6NH−C∣∣O−(CH2)4−C∣∣O⎫⎪ ⎪⎬⎪ ⎪⎭n is a
AHomopolymerBCopolymerCAdditioin polymerDThermosetting polymerThe IUPAC name of compound
C | CHOH3−C | CHOH−C | CHOH2 isA3-Formyl-1,5-pentanedialB1,2,3-TriformylpropaneCPropanetrialDPropane-1,2,3-tricarbaldehyde
CH3−C∣ClH−C∣IH−CH3
CH3−C∣CH3H−(CH2)4−C∣CH3H−C∣BrH−CH2CH3
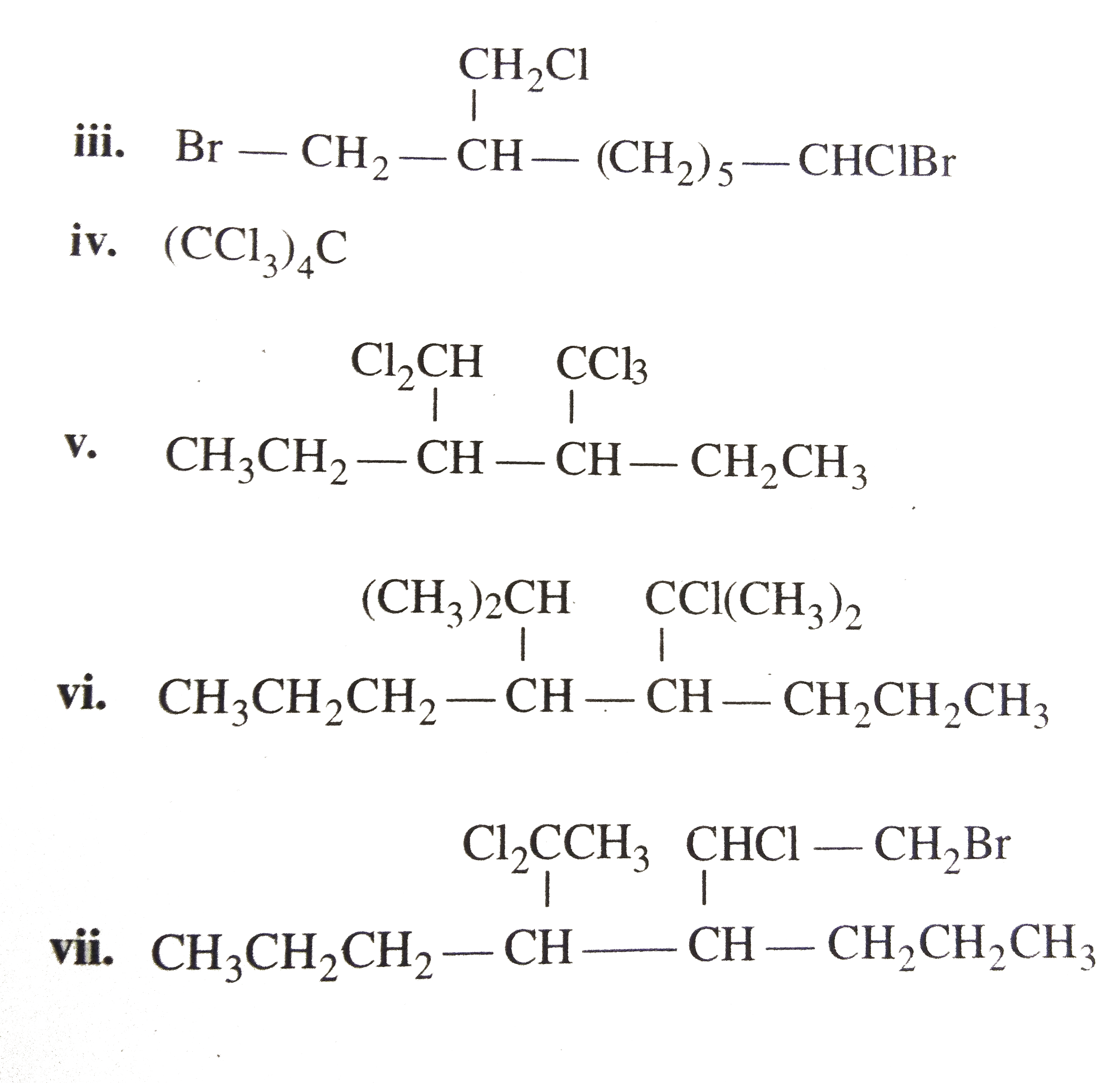
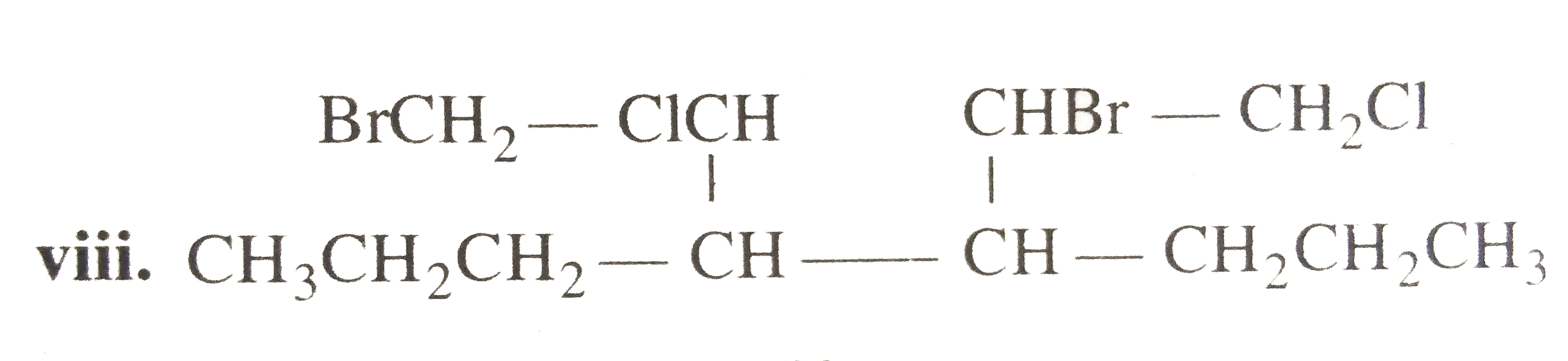
View SolutionGiven below are the sets of quantum numbers for given orbitals .Name these orbitals
a.n = 2 l = 1 m = -1<br>b.n = 4 l = 2 m = 0<br>c.n = 3 l = 1 m = +- 1<br>d.n = 4 l = 0 m = 0<br>e.n = 3 l = 2 m = +- 2
View SolutionIUPAC name of the compound given below is -
CH3−C≡C−C∣CH3H−C∣CH2∣CH3=CH2
View SolutionStudent I,II,andIII perform an experiment for measuring the acceleration due to gravity (g) usinf a simple pendulum. They use lengths of the pendulum and // or record time for different number of oscillations . The observations are shown in the following table . Least count for length =0.1cm
StudentIIIIIILength of Pendulam(cm)64.064.020.0Number ofn Oscillation(n)844TimePeriod(s)16.016.09.0
Least count for time =0.1s.
If EI,EII , and EIII are the percentage errors in g , i.,e., (Δgg×100) for students I,II , and III, respectively , then
View SolutionSelect the IUPAC name of the following
C2H5−N∣C2H5−O∣∣S∣∣O−C6H5
View Solution

