Calculate ΔG for the reaction at 25∘C when partial pressure of reactants H2, CO2, H2O and CO are 10, 20, 0.02 and 0.01atm respectively.
H2(g)+CO2(g)⇔H2O(g)+CO(g)
Given G∘f for H2(g), CO2(g), H2O(g) and CO(g) are 0, −394.37, −228.58, −137.15KJ respectively.
5.61kJ ,
Similar Questions
Calculate ΔfH∘ for the reaction,
CO2(g)+H2(g)→CO(g)+H2O(g) Given that ΔfH∘ for CO2(g), CO(g) and H2O(g) are -393.5, -111.3 and −241.8kJmol−1 respectively. ( Round of your answer in two digits and in KJ)
View SolutionCalculate the equilibrium constant for the reaction,
H2(g)+CO2(g)⇔H2O(g)+CO(g) at 1395K, if the
equilibrium constants at 1395K for the following are:
2H2O(g)⇔2H2+O2(g) (K1=2.1×10−13)
2CO2(g)⇔2CO(g)+O2(g) (K2=1.4×10−12)
View Solution
Knowledge Check
What will be ΔG for the reaction at 25∘C when partial pressures of reactants H2,CO2,H2OandCO are 10, 20, 0.02 and 0.01 atm respectively. (Given : G∘H2O=−228.58 kJ, G∘CO=−137,15 kJ, G∘CO=−394.37 kJ.)
A+5.61kJB−5.61kJC7.09kJD−8.13kJWhich of the following graphs are correct for the given reaction?
H2(g)+CO2(g)⇔H2O(g)+CO(g)
Assume initially only H2 and CO2 are present:A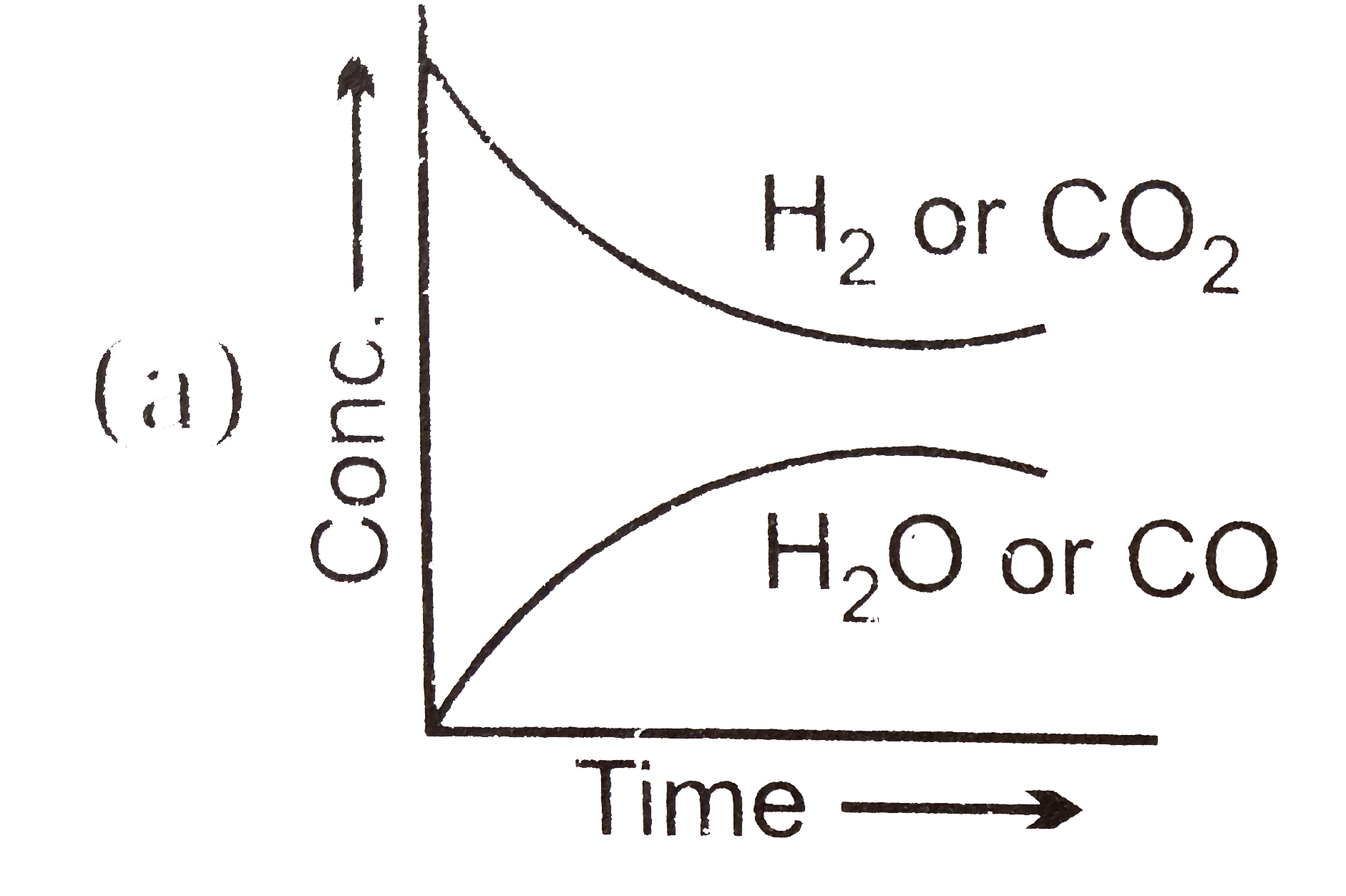 B
B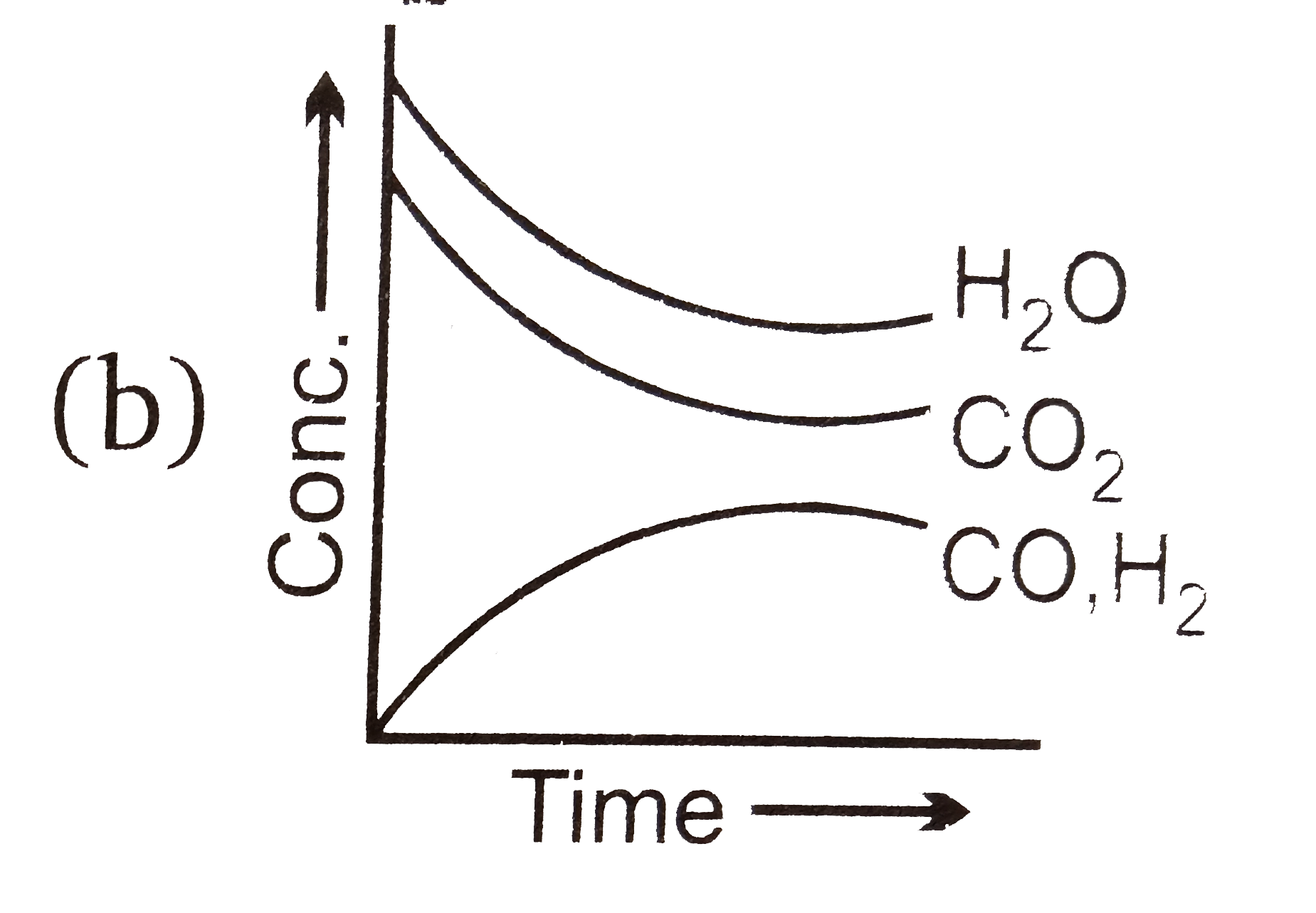 C
C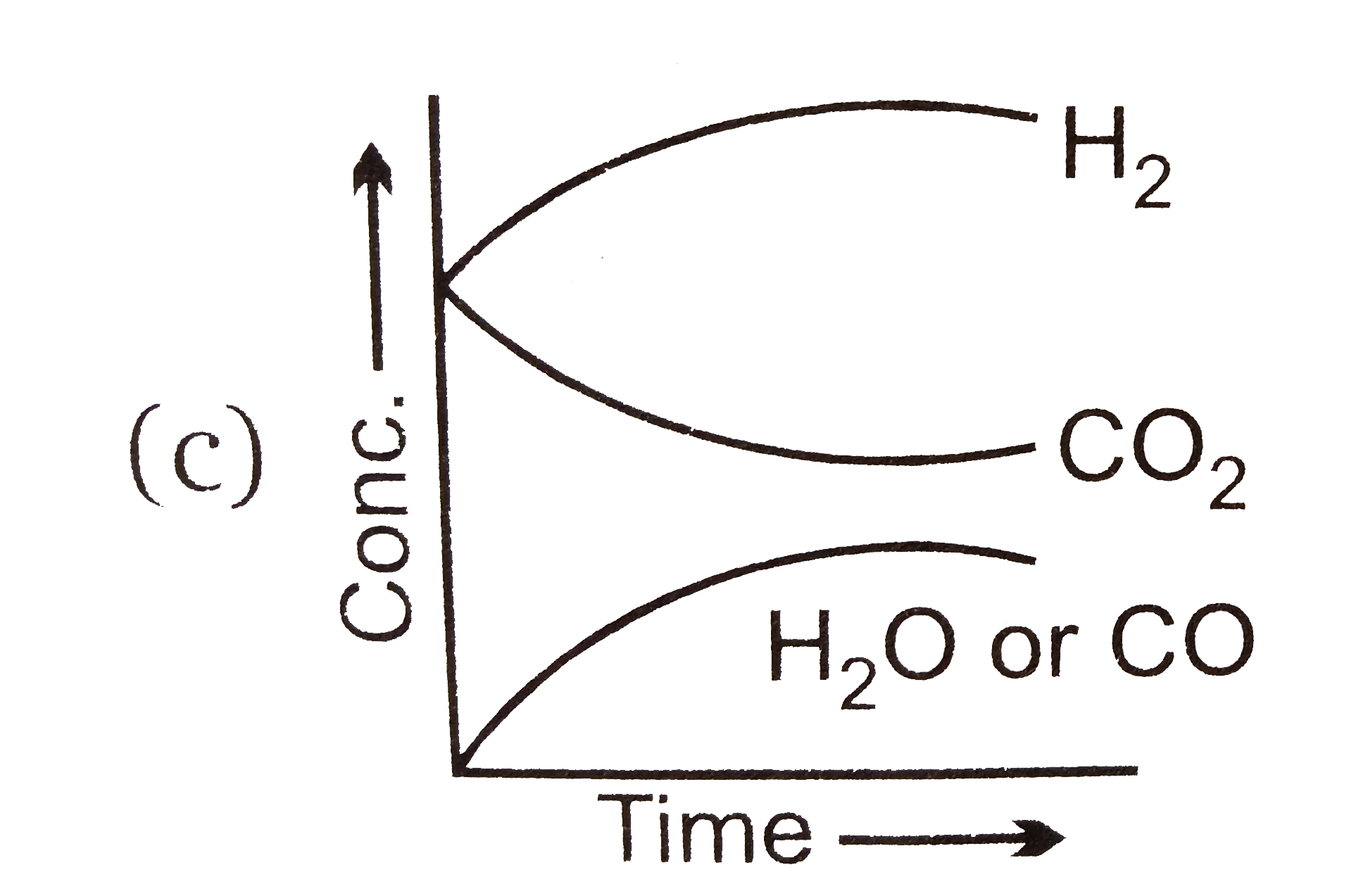 D
D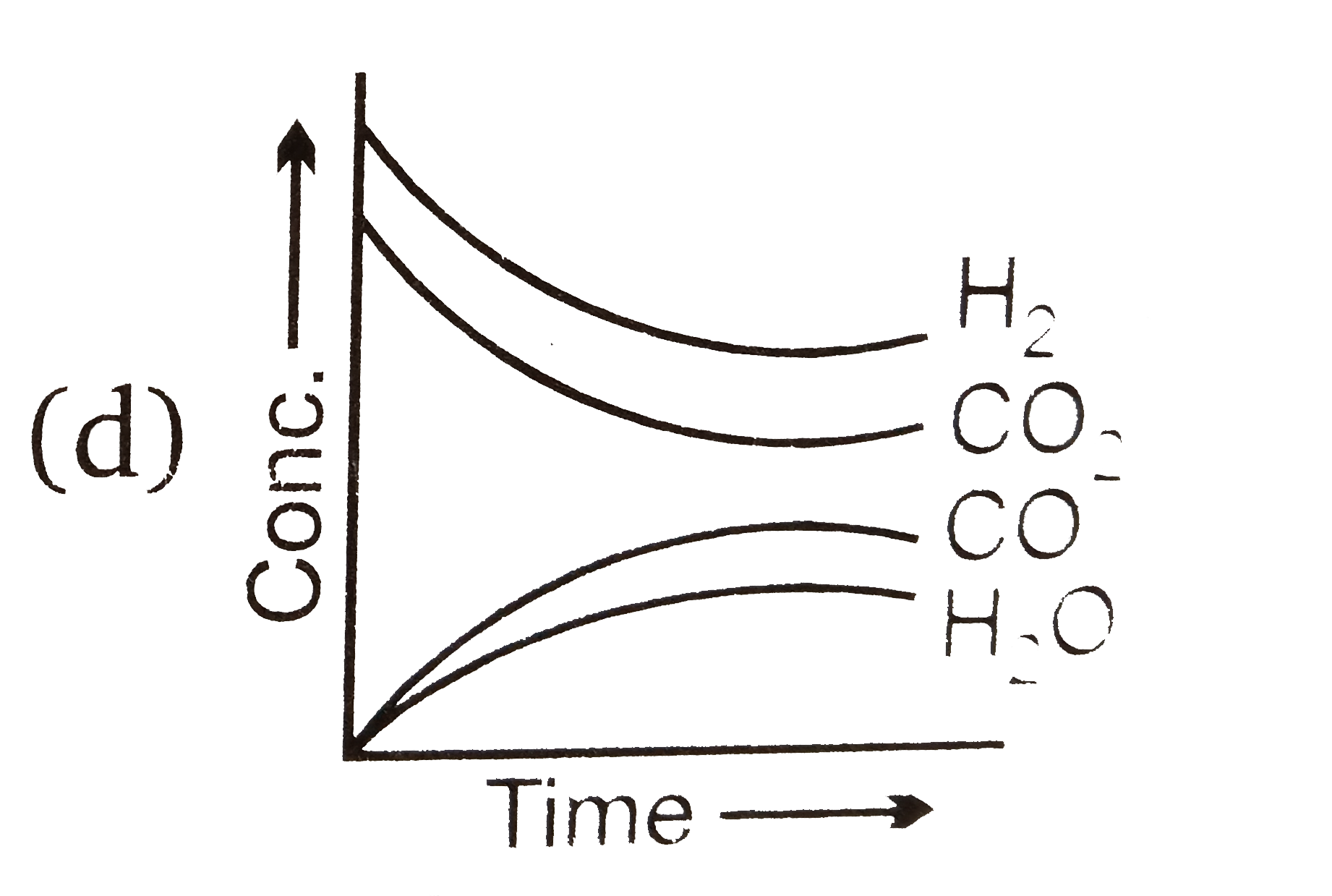
Δ∘f for CO2(g),CO(g) and H2O(g) are −393.5,−110.5 and −241.8kJmol−1 respectively. The standard enthalpy change ( in kJ) for given reaction is :
CO2(g)+H2(g)→CO(g)+H2O(g)A+524.1B+41.2C−262.5D−41.2
The ΔH∘ for CO2(g),CO(g) and H2O(g) are -393.5, -110.5 and −241.8kJmol−1 respectively.The standard enthalpy change (in kJ) for the reaction :
CO2(g)+H2(g)→CO(g)+H2O(g) is
View Solutionthe ΔH∘tforCO2(g),CO(g)andH2O(g)are−393.5,−110.5and−241.8kJmol−1 respectively, the standed enthalpy change ( in kJ) for the reaction
CO2(g)+H2(g)→CO(g)+H2O(g) is
View SolutionThe ΔH∘t for CO2(g), CO(g) and H2O (g) are −393.5,−110.5 and −241.8kJmol−1 respectively. The standard enthalpy change (in kJ) for the reaction.
CO2(g)+H2(g)→CO(g)+H2O(g) is
View SolutionΔH∘1 for CO2(g), CO(g) and H2O(g) are −393.5,−110.5 and −241.8kJmol−1 respectively. Standard enthalpy change for the reacion
CO2(g)+H2(g)→CO(g)+H2O(g) is
View SolutionFor the following three reaction a, b and c, equilibrium constants are given :
(i) CO(g)⋅H2Og⇔CO2(g)⋅H2(g),K1
(ii) CH4(g)⋅H2O(g)⇔CO(g)⋅3H2(g),K2
(iii)CH4(g)+2H2O(g)⇔CO2(g)+4H2(g),K3
View Solution
