How many line pair of electrons are present in xeF4 ?
Video Solution
Text Solution
Xe is in sp3d2 -hybridised state having ( 2) lone pair of electrons .
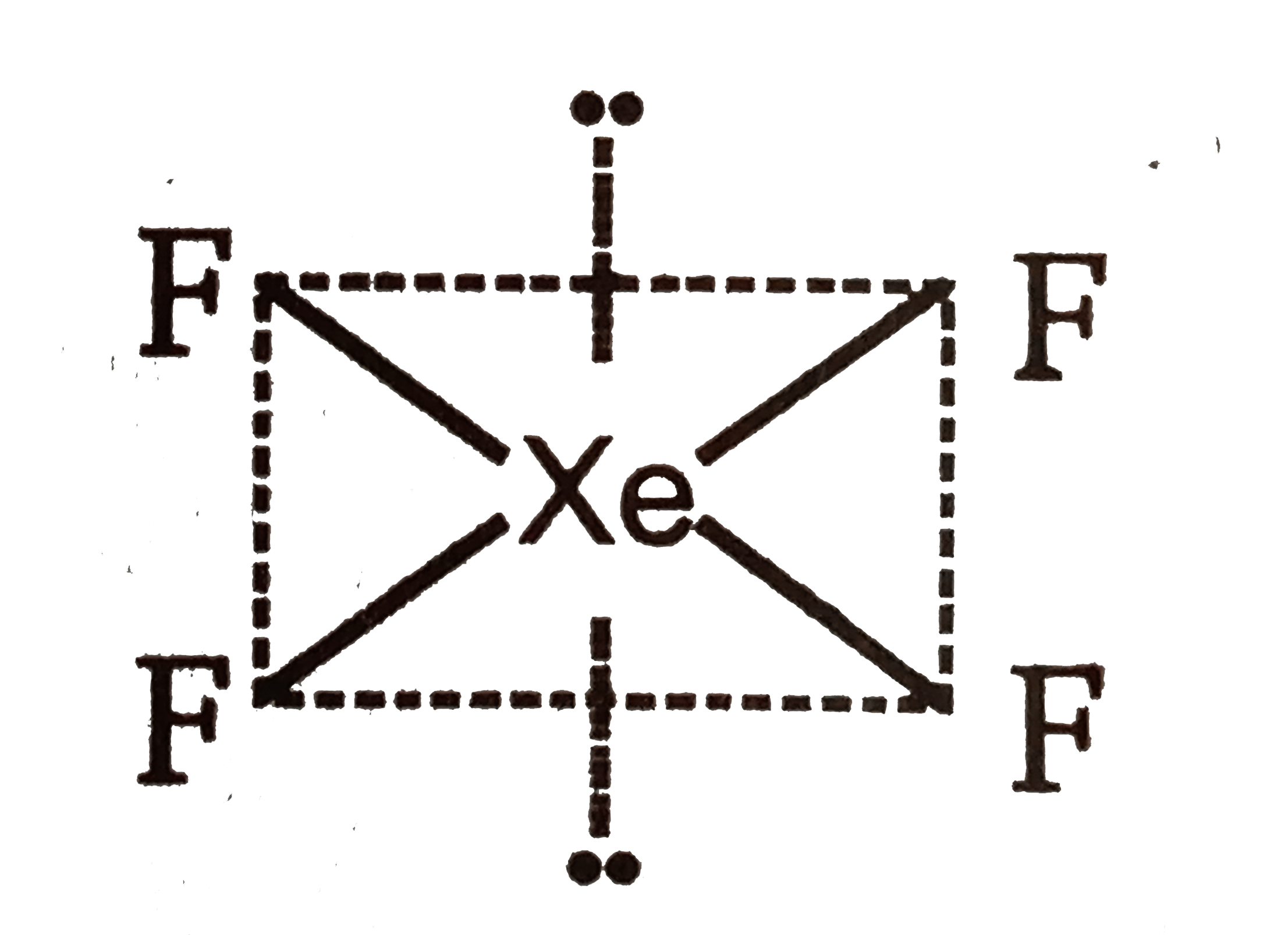
|
Similar Questions
Knowledge Check
How many unpaired electrons are present in N+2 ?
A1B2C3D4How many lone pair of electrons are present on chlorine in ClF3 molecule ?
A0B1C2D3How many unshared pair of electrons are present in water molecule?
AOneBZeroCTwoDThree
How many lose pair of electrons are present on the central atom of CH4,H2O,NH3,PCl3 and PCl5 molecules?
View SolutionHow many unpaired electrons are present in Ni2+?
View SolutionHow many upaired electrons are present in Ni2+?
View SolutionHow many unpaired electrons are present in a ground state?
View SolutionIn the Lewis structure for formic acid, HCOOH, how many bonding pairs and lone pairs of electrons are present?
View Solution
