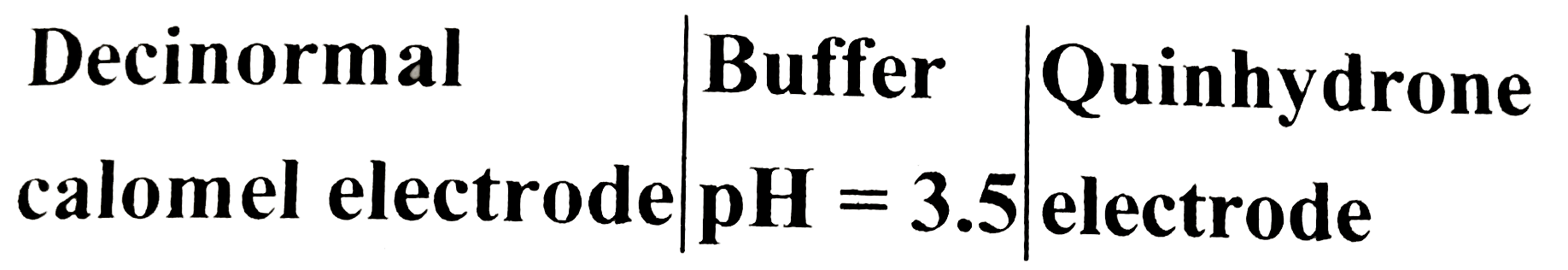
At pH=2,E∘(Quinhydrone)=1.30V,EQuinhydrone will be :
A
1.36V
B
1.30V
C
1.42V
D
1.20V
Video Solution
Text Solution
The correct Answer is:C
E=E∘=0.0592log[H+]2
=1.30−0.0592log(10−2)2
=1.30+0.2362=1.418V
|
Similar Questions
Knowledge Check
At pH=2,E∘(Quinhydrone)=1.30V,EQuinhydrone will be :
 A1.36 VB1.32 VC1.42 VD1.26 V
A1.36 VB1.32 VC1.42 VD1.26 VThe term quinhydrone means
Aa quinoneBa hydroquinoneCa mixture of quinone and hydroquinoneDnone of abovepH = 2, E_("क्विनहाइड्रोन")^(0) = 1:30V, E_("क्विनहाइड्रोन") होगा:
 A1.36VB1.32VC1.42VD1.26V
A1.36VB1.32VC1.42VD1.26V
If E∘Au+/Au is 1.69 V and E∘Au3+/Au is 1.40 V, then E∘Au+/Au3+ will be :
View SolutionThe E∘Ag=0.80V and E∘cell=1.20 V , for the cell .
2Ag++Cd→2Ag+Cd2+ , the EoCd is
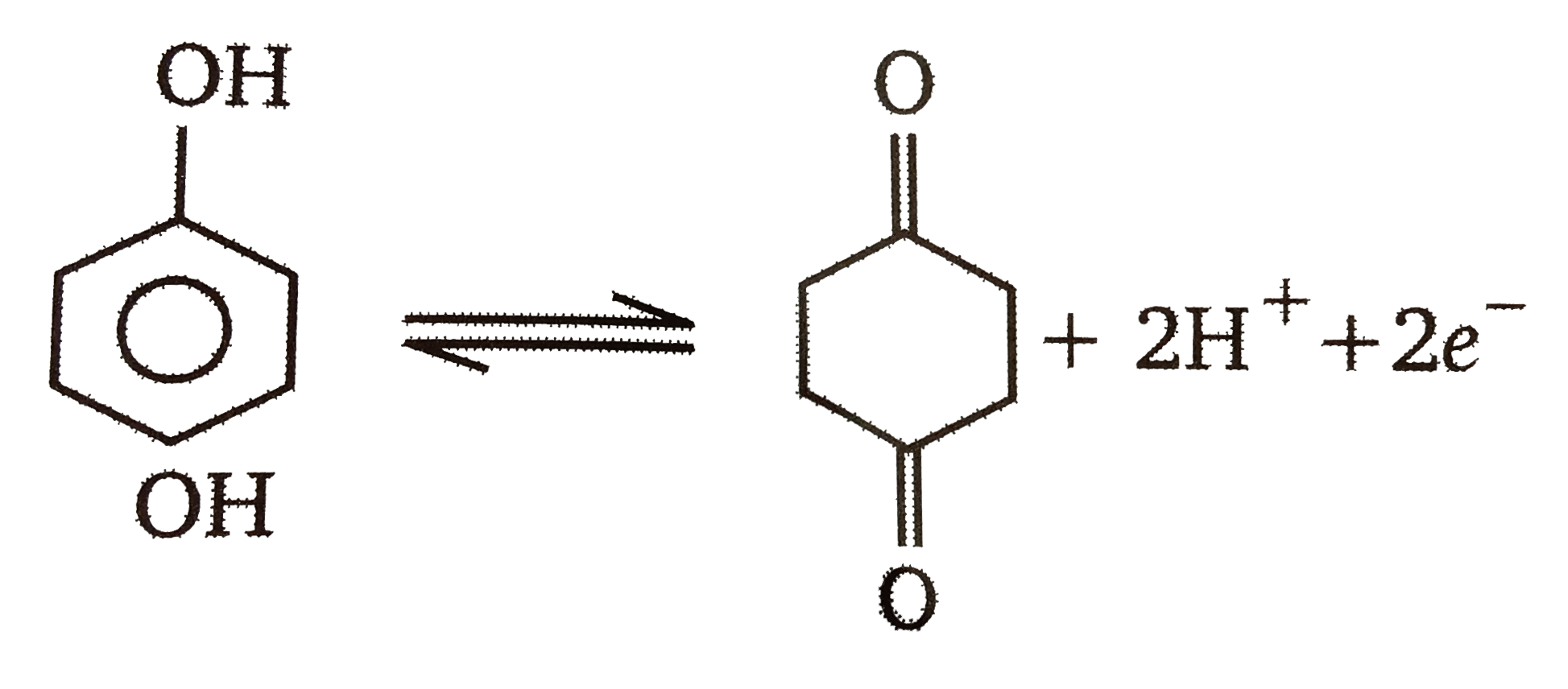
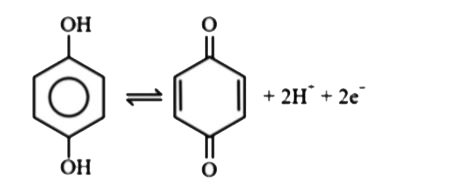
View SolutionFor the half cell,
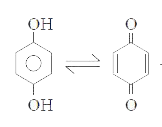
+2H+2e−.E0=
1.30 V. At pH=2, electrode potential of the given quinhydrone half cell is :
View SolutionThe satandard EMF of quinhydrone is 0.699V. The EMF of the quinhydrone electrode dipped in a solution with pH=10 is
View SolutionGiven ECr3+/Cr∘=−O⋅74V,E∘MnO−4/Mn2+=1.51V
E∘Cr2O2−7/Cr3+= 1.33V , E∘Cl/Cl−=1.36V
Based on the given above , Strongest oxidising agent will be:
View Solution