The emf of given cell Pt−H2P1∣∣H+∣∣H2−PtP2 is :
A
RTFlog'P1P2
B
RT2Floge(P1)P2
C
RTFloge(P2)P1
D
None of these
Video Solution
Text Solution
The correct Answer is:B
H2P1→2H++2e
2H++2e→H2P2
Ecell=E∘OPH+E∘RPH+Rt2Floge[PH2]1[PH2]2
=Rt2Floge(P1)P2
|
Similar Questions
Knowledge Check
What will be the emf of given cell -
Pt|H2(P1)|H+(aq)∣|H2(P2)|Pt ?ART2FlogeP1P2BRTFlogeP1P2CRTFlogeP2P1DRT2FlogeP2P1For the given cell,
Pt,C12P1∣∣∣HC1C1∣∣∣∣∣∣HC1C2∣∣∣C12,PtP2
The emf will be positive when :AC1>C2BC2>C1CC1=C2DNone of theseThe e.m.f. of the followig cell,
Zn∣∣∣ZnSO4(1M)∣∣∣∣∣∣H+(1M)∣∣∣H2g1atm∣Pt is 0.769 volts
The reduction potential of Zn electrode will beAzero voltB0.769 VC−0.769VD7.69V
The emf of given cell Pt−H2∣∣H+∣∣H2−Pt is :
View SolutionWhat will be the emf for the given cell ?
Pt|H2(g,P1)|H+(aq)|H2(g,P2)|Pt
View SolutionWhat will be the emf for the given cell ?
Pt|H2(g,P1)|H+(aq)|H2(g,P2)|Pt
View Solution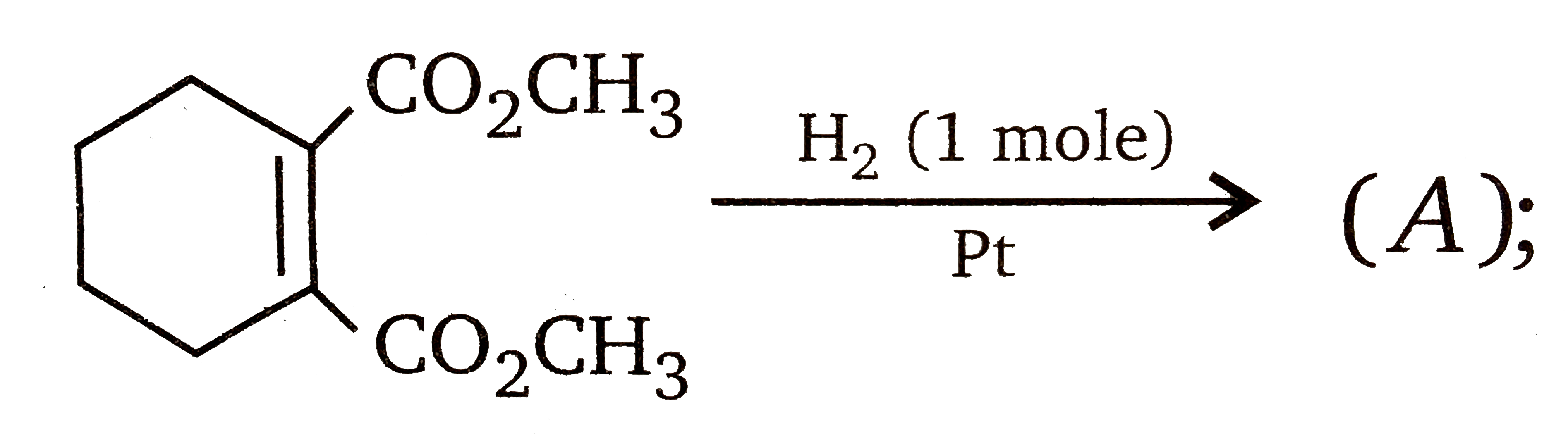 H2(1 mole)−−−−−−−−→Pt(A), product (A) is:
H2(1 mole)−−−−−−−−→Pt(A), product (A) is:
View Solution (i)Br2,H+−−−−−−−−→(ii)C6H5OnaP,P will be
(i)Br2,H+−−−−−−−−→(ii)C6H5OnaP,P will be
View Solution

