For the following first order parallel chain reaction. If the half-life of A is 25 seconds, then find out the half-life of B,C and D. Given that the rate of formation of B and C is 15% and 30% of the rate of decomposition of A respectively.
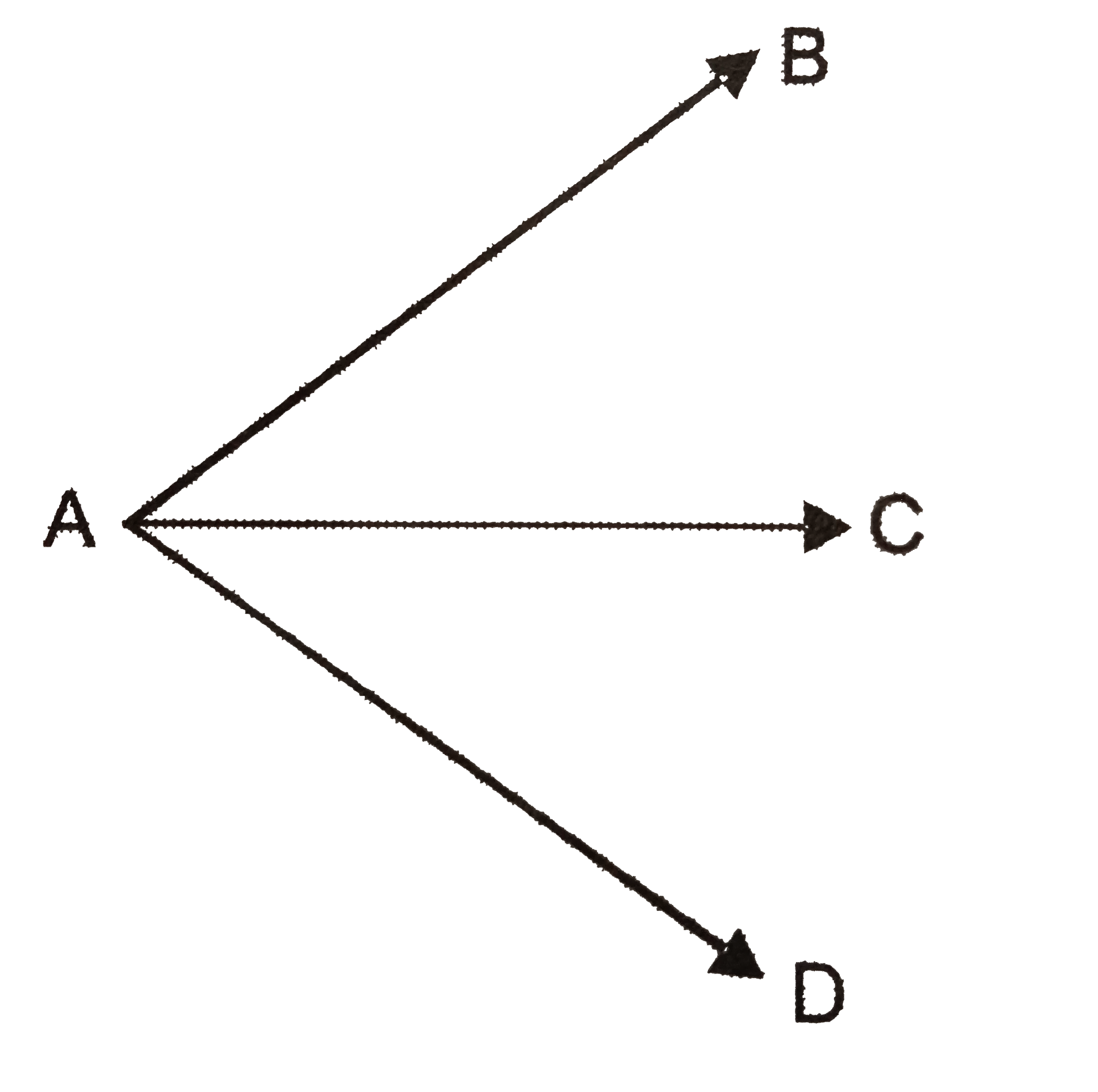
Let the rate constants for the formation of B,C and D are K1,K2,K3 and overall rate constant is K.
K=K1+K2+K3
∵K=0.693t1/2=0.69325s−1
∴K1=0.15K
K1=0.15×0.69325
⇒t(1/2)=0.693K1=0.6930.15×0.69325
⇒t(1/2)=250.15s=166.66sec
Similarly, t(1/2)2=250.3s=333.33sec
and t(1/2)3=250.55s=45.45sec
Similar Questions
Consider the following parallel first order reactions
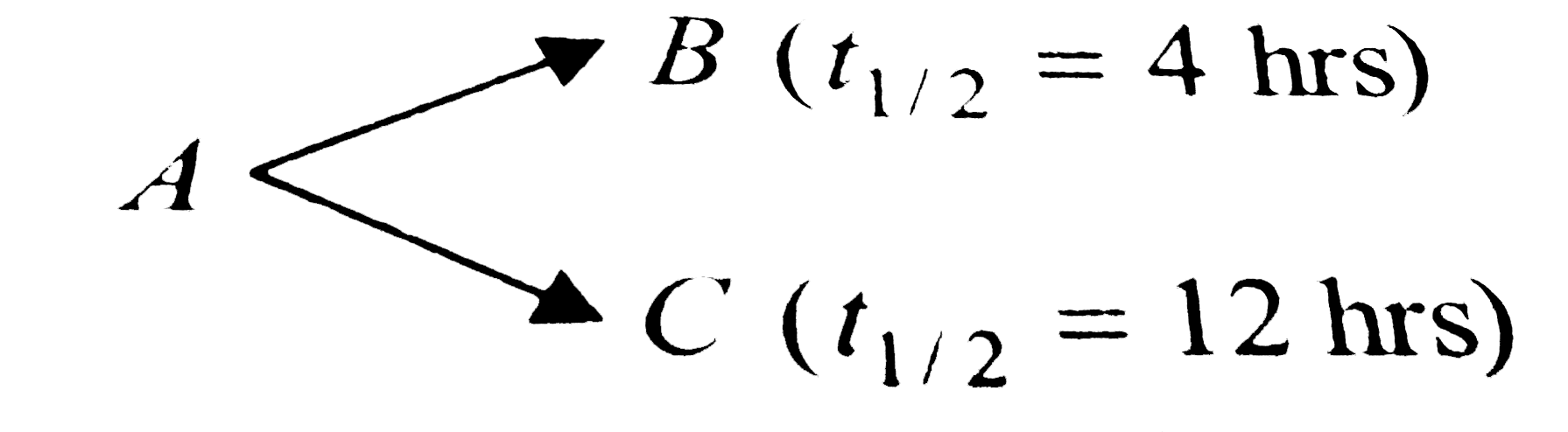
The half-life for the decay of A is ........hrs.
View SolutionConsider the following parallel first order reactions:
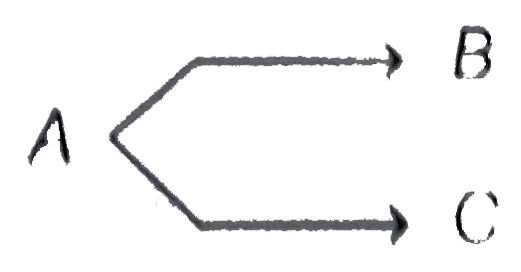
Half-life of the overall decay of A is 2 ys while half-life for the formation of B is 3yr. Half-life for the formation for C (in years) is :
View Solution
Knowledge Check
A first order reaction has a half-life period of 34.65 seconds. Its rate constant is
A2×10−2sec−1B4×10−4sec−1C20sec−1D2×10−4sec−1The rate constant for a first order reaction whose half life is 480 sec, is :
A2.88×10−3sec−1B1.44×10−3sec−1C1.44sec−1D0.72×10−3sec−1The rate constant of first order reaction whose half-life is 480 s, is
A1.44×10−3s−1B1.44s−1C0.72×10−3s−1D2.88×10−3s−1
A first order reaction in 75% complete is 60 minutes. Find the half life of this reaction.
View SolutionFor a first order reaction A→ Products, the half life is 100 seconds. The rate constant of the reaction is
View SolutionFor a first order reaction A→ Products, the half life is 100 seconds. The rate constant of the reaction is
View Solution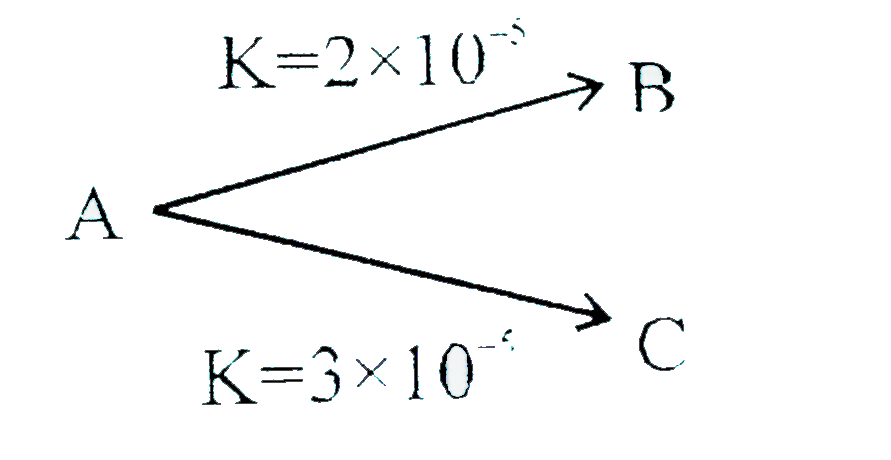
For this parallel first order reaction then find out percentage of B = ?
View SolutionThe rate constant for a first order reaction whose half life is 480 sec, is :
View Solution
