Statement-1L The bond angles in molecules depneds upon hybridization electronagativity of central atom, no. of lone pair, odd electron and multiplicity of bond.
Statement-2 NO2 and NO−2 have angles 134∘ and 115∘ respectively.
If both the statement are TRUE and Statement -2 is the correct explanation of Statement-1:
If both the statement are TRUE but Statement-2 is not the correct explanation of Statement-1
If statement-1 is TRUE and Statement-2 is FALSE
If statement -1 is FALSE and Statement-2 is TRUE
Explanation is correct reason for statement.
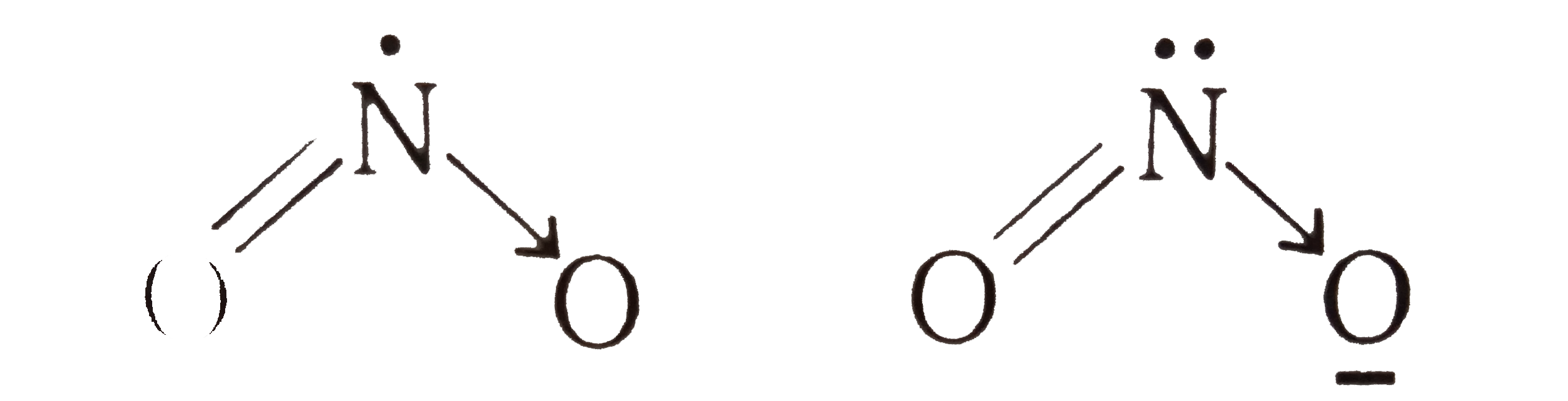
Similar Questions
In NO_(3)^(-) ion, the number of bond pairs and lone pairs of electrons on nitrogen atom are :
View SolutionWhat are the number of bond pairs and lone pairs of electrons on N - atom in NO−3 ?
View SolutionWhich has highest bond angle? NO_(2), NO_(2)^(–) , NO_(2)^(+)
View SolutionArrange the following in the order of property indicated for each set: NO_(2), NO_(2)^(+), NO_(2)^(–) (decreasing bond angle)
View SolutionWhich has highest bond angle? NO_(2), NO_(2)^(–), NO_(2)^(+)
View SolutionMolecule which contains 4 bond pairs and 2 lone pairs of electrons on the central atom is
View SolutionWith increase in lone pair on Central atom, Bond angle
View Solution
बंध कोण P = बंध कोण Q लेकिन निश्चित 90∘ नहीं।
कथन 2 : अणु T-आकृति का थोड़ा झुका हुआ तथा इलेक्ट्रॉनों के एकाकी युग्मों के बीच प्रतिकर्षण होता है ।
View Solution

