The heat is supplied to a given mass of water. Draw the variation of volume of water with its temperature.
The variation of given volume of one kg of water (V) with temperatrue (t∘C) .
Similar Questions
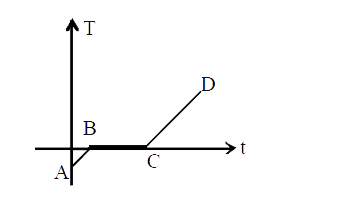
Heat is supplied to a ice at a constant rate Temperature variation with time is as shown in figure. Then –
View SolutionThe molar heat capacity of water at constant pressure P is 60JK−1mol−1. When 1.0kJ of heat is supplied to 100g of water, which is free to expand, find the increase in temperature of water.
View Solution
Knowledge Check
The variation of heat of reaction with temperature is given
AVan't Hoff equationBClausius-Clapeyron equationCNernst equationDKirchoff's equationIf heat a supplied to a solid, its temperature
Amust increaseBmay increaseCmay remain constantDmay decreaseVariation of heat of reaction with temperature is known as
AVant Hoff's isothermBVant Hoff's isochoreCKirchoff's equationDKemmerling equation
When heat Q is supplied to a diatomic gas of rigid molecules, at constant volume its temperature increases by ΔT. The heat required to produce the same change in temperature, at a constant pressure is , where xQ is ___________ .
View SolutionThe molar heat capacity of water at constant pressure is 75 JK−1mol−1. When 1.0 kJ of heat is supplied to 100 g of water which is free to expand the increase in temperature of water is
View SolutionThe molar heat capacity of water at constant pressure, CP , is 75JK−1mol−1. When 1.0kJ of heat is supplied to 100g of water which is free to expand, the increase in temperature of water is
View SolutionThe molar heat capacity of water at constant pressure P is 75JK−1mol−1. When 1.0kJ of heat is supplied to 1000 g of water , which is free to expand, the increase in temperature of water is
View SolutionThe molar heat capacity of water at constant pressure, CP , is 75JK−1mol−1. When 1.0kJ of heat is supplied to 100g of water which is free to expand, the increase in temperature of water is
View Solution

