What is an isobaric process ?
Step-by-Step Solution:
1. Definition of Isobaric Process:
- An isobaric process is a thermodynamic process in which the pressure remains constant throughout the entire process. The term "isobaric" comes from the Greek words "iso," meaning equal or constant, and "baros," meaning pressure.
2. Understanding the Graph:
- In an isobaric process, if we plot a graph with pressure on the y-axis and volume on the x-axis, the graph will be a horizontal line. This indicates that as the volume changes, the pressure does not change.
3. Relationship with Temperature:
- According to the ideal gas law, PV=nRT, if the pressure (P) is constant, then any change in volume (V) will directly affect the temperature (T). Specifically, if the volume increases, the temperature must also increase, and vice versa, to maintain the equation's balance.
4. Conclusion:
- Therefore, an isobaric process is characterized by constant pressure, and it can be represented graphically as a horizontal line on a pressure-volume (P-V) diagram. This process is significant in various thermodynamic applications, such as heating or cooling gases at constant pressure.
- An isobaric process is a thermodynamic process in which the pressure remains constant throughout the entire process. The term "isobaric" comes from the Greek words "iso," meaning equal or constant, and "baros," meaning pressure.
- In an isobaric process, if we plot a graph with pressure on the y-axis and volume on the x-axis, the graph will be a horizontal line. This indicates that as the volume changes, the pressure does not change.
- According to the ideal gas law, PV=nRT, if the pressure (P) is constant, then any change in volume (V) will directly affect the temperature (T). Specifically, if the volume increases, the temperature must also increase, and vice versa, to maintain the equation's balance.
- Therefore, an isobaric process is characterized by constant pressure, and it can be represented graphically as a horizontal line on a pressure-volume (P-V) diagram. This process is significant in various thermodynamic applications, such as heating or cooling gases at constant pressure.
Similar Questions
Knowledge Check
In isobaric process
Asize of the bar remains sameBsize of barometer remains sameCpressure of the system remains sameDvolume remains sameIn an isobaric process, __________ .
Asize of the bar remains sameBsize of barometer remains sameCpressure of the system remains sameDvolume remains sameIn an isobaric process
APressure increasesBPressure decreasesCPressure neither increases or decreases (P fixed),DNone of these.
reversible & irreversible process,comparison OF work done in reversible & irreversible process,isochoric and isobaric process
View SolutionWhen heat in given to a gas in an isobaric process, then
View SolutionChoose the incorrect statement related to an isobaric process
View Solution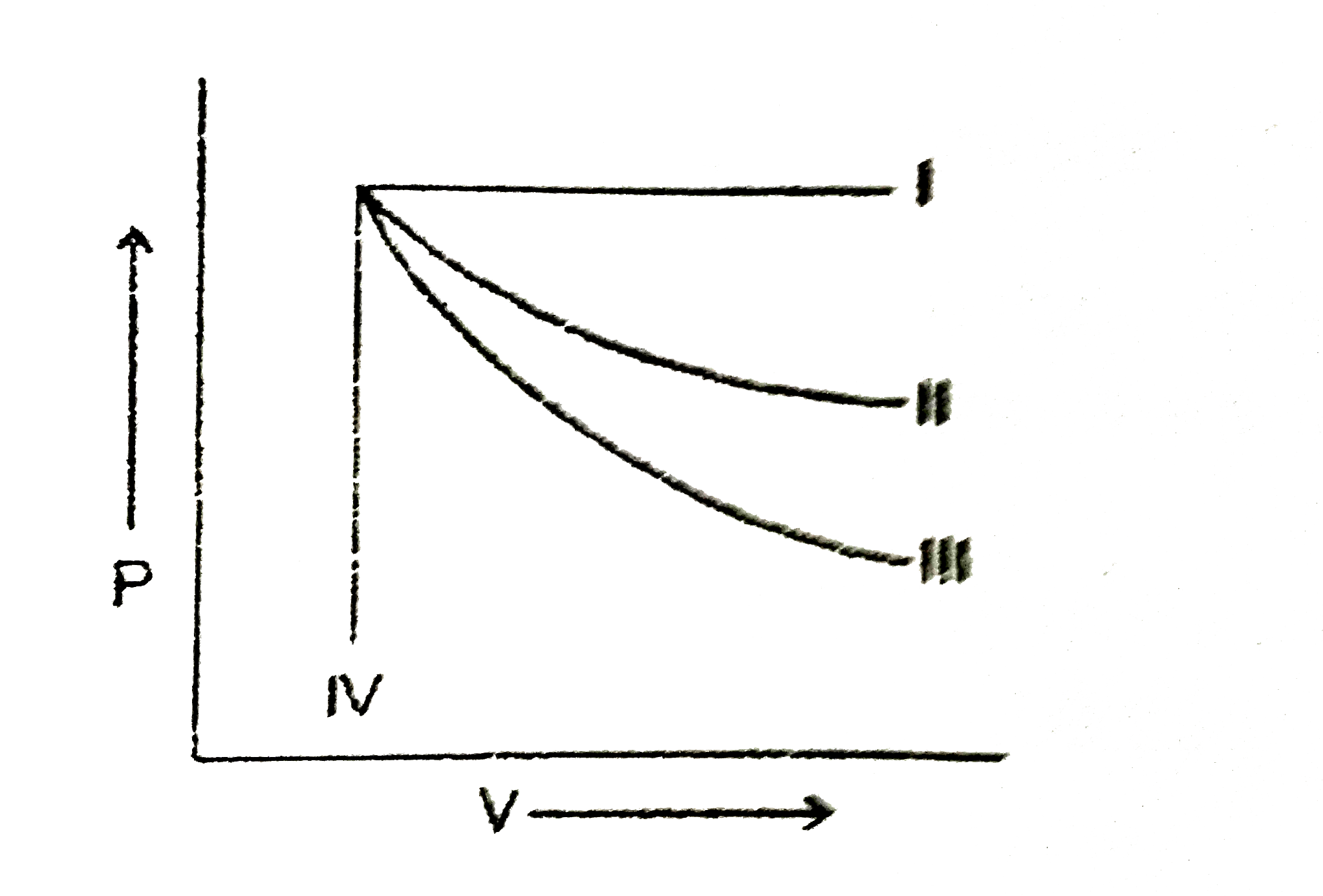
The plots between P and V which represent isochoric and isobaric process respectively:
View SolutionWhich of the following is state function (a)q+w,(b)q(c )w(d) heating isobaric process (e) work in adiabatic process :-
View Solution

