The frequency (v) of the incident radiation is greater than threshold frequency v0 in photocell. How will the stopping potential very if frequency v is increased, keeping other factors constant.
As, eV0=h(v−v0), therefore, the value of stopping potential (V0) increases with the increase in frequency (v) of the incident radiation.
Similar Questions
Knowledge Check
Increase in the frequency of the incident radiations increases the
Arate of emission of photoelectronsBwork functionCkinetic energy of photoelectronsDthreshold frequency.In aphotocell frequency of incident radiation is increased by keeping other factors constant (v>v0), the stopping potential
AdecreasesBincreasesCbecomes zeroDfirst decreases and then increasesLight of frequency v is incident on a substance of threshold frequency v(v<v). The energy of the emitted photo-electron will be
Ah(v−v0)Bh/vChe(v−v0)Dh/v0
If the intensity of the incident radiation in a photocell is increased, how does the stopping potential very?
View SolutionWrite Einstein’s photoelectric equation. Define the threshold frequency v0 and stopping potential V0.
View SolutionPlot a graph showing variation of stopping potential (V0) with the frequency (v) of the incident radiation for a given photosensitive material. Hence, state the significance of the threshold frequency in photoelectric emission.
Using the principle of energy conservation, write the equation relating the energy of incident photon, threshold frequency and the maximum kinetic energy of the emitted photoelectrons.
View SolutionUsing the graph shown in fig for stopping potential vs the incident frequency of photons, calculate Planck's constant.
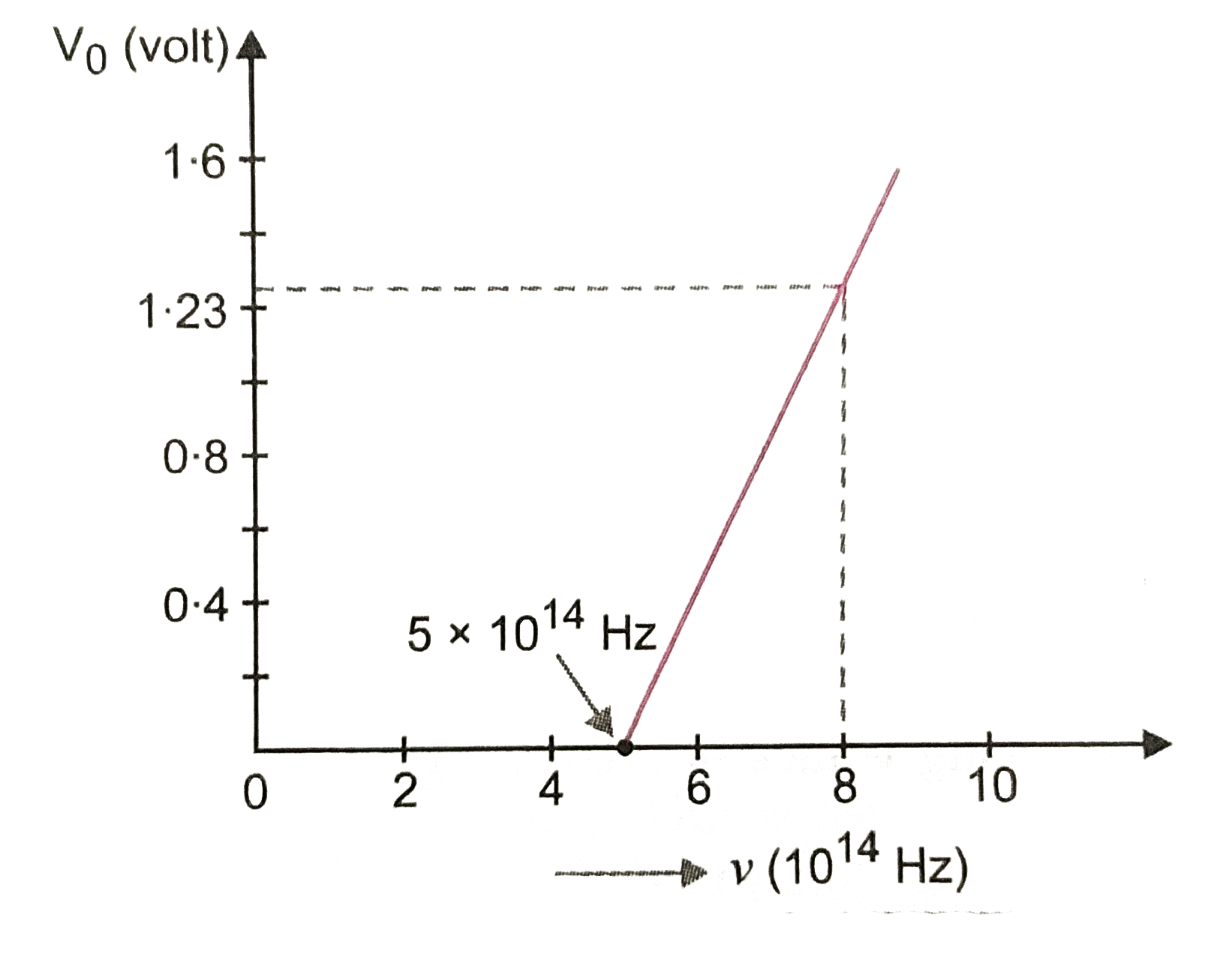
View SolutionLight of frequency v is incident on a certain photoelectric substance with threshold frequency v. The work function for the substance is
View Solution
