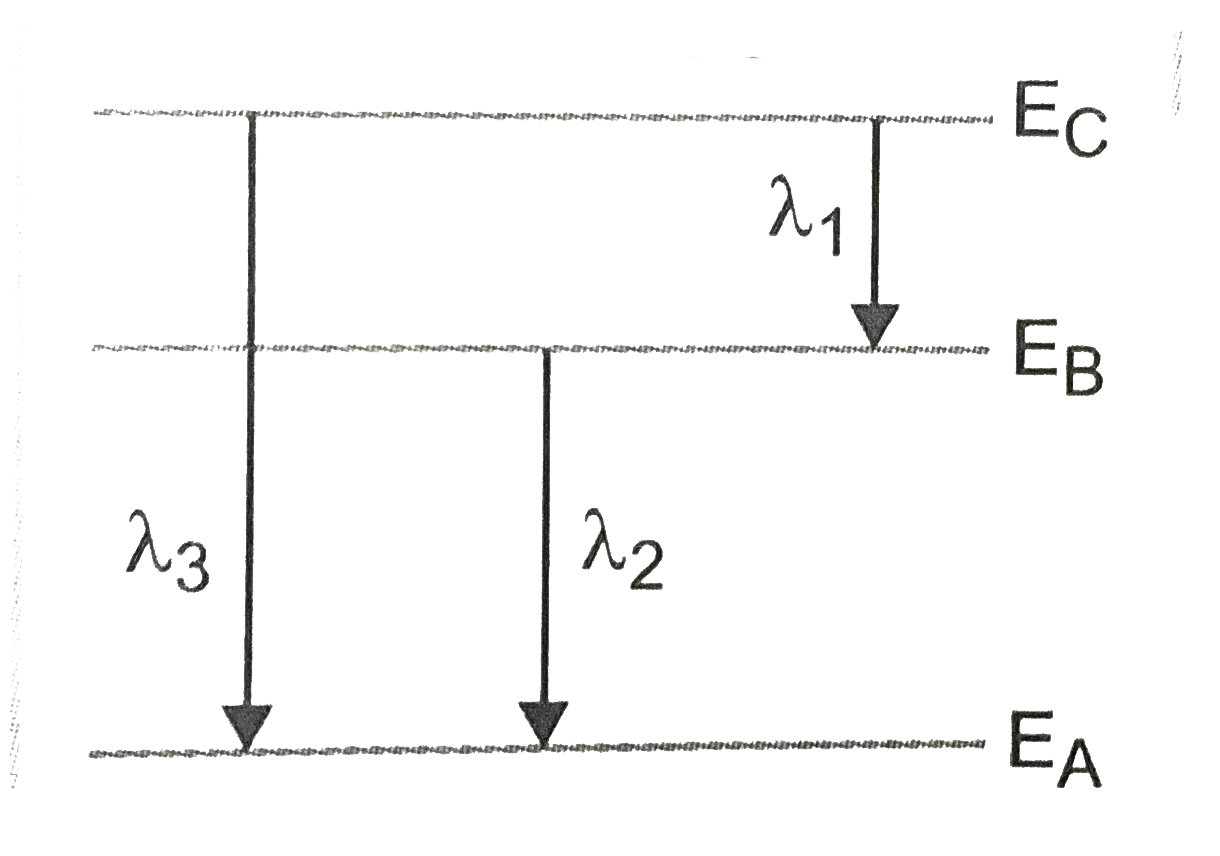
For an atom of an ion having single electron, the wavelengths observed are λ1=2unitsandλ3=3units. The value of missing wavelength λ2 is:
Video Solution
Text Solution
The correct Answer is:6
As is clear form fig.
E1=E2+E3
hcλ1=hcλ2+hcλ3
∴1λ1=1λ2−1λ3=12−13=16∴λ2=6units
|
Similar Questions
Knowledge Check
The wavelength of electron waves in two orbits (λ1:λ2)
A25:9B5:3C9:25D3:5A dye absorbs a photon of wavelength λ and re− emits the same energy into two phorons of wavelengths λ1 and λ2 respectively. The wavelength λ is related with λ1 and λ2 as :
Aλ=λ1+λ2λ1λ2Bλ=λ1λ2λ1+λ2Cλ=λ21λ22λ1+λ2Dλ=λ1λ2(λ1+λ2)2The ratio of resolving power of an optical microscope for two wavelength λ1=4000Å and λ2=6000Å is:
A9:4B3:2C16:81D8:27
Let be the maximum kinetic energy of photoelectrons emitted by light of wavelength λ1 and λ2 correspondingto wavelength λ2. If λ1=2λ2 then:
View SolutionBichromatic light is used in YDSE having wavelengths λ1=400nm and λ2=700nm Find minimum order of λ1 which overlaps with λ2
View SolutionThe ratio of resolving power of an optical microscope for two wavelength λ1=4000Å and λ2=6000Å is:
View SolutionAn electron in a hydrogen like atom makes transition from a state in which itd de Broglie wavelength is λ1 to a state where its de Broglie wavelength is λ2 then wavelength of photon (λ) generated will be :
View SolutionLast line of Lyman series for H− atom has wavelength λ1A,2nd line of Balmer series has wavelength λ2A then
View Solution
