Molecularity of an elementary reaction
may be zero
is always integral
may be semi - integral
may be integral fractional or zero
The correct Answer is:B
Step by step video, text & image solution for Molecularity of an elementary reaction by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Determining the overall reaction and the molecularity of an elementary reaction : The following two - step mechanism hqas proposed for the gas-phase decomposition of nitrous oxide (N2O) :
Step 1: N2O(g)→N2(g)+O(g)
Step 2: N2O(g)+O(g)→N2(g)+O2(g)
(i) Write the chemical equation for the overall reaction
(ii) Identify any reaction intermediates
(iii) What is the molecularity of each of the elementary reactions ?
(iv) What is the molecularity of the overvall reaction
Strategy : The step-by -step pathway by which a reaction occurs is called its mechanism. Some reactions takes place in a single step, but most reactions occur in a series of elementary steps. To identify intermediates and molecularity, look at the indicidual steps
View SolutionWhich of the following statements are applicable to a balanced chemical equation of an elementary reaction?
(a) Order is same as molecularity
(b) Order is less than the molecularity
(c) Order is greater than the molecularity
(d) Molecularity can never be zero .
View Solution
Knowledge Check
In question no. (23) as given above, what would be the molecularity of elementary reaction [a] and [b] respectively?
A3,3B2,2C1,1D3,2Which of the following statements are applicable to a balanced chemical equation of an elementary reaction ?
AOrder is same as molecularityBOrder is less than the molecularityCOrder is greater than the molecularityDMolecularity can never he zeroWhich of the following statements are applicable to a balanced chemical equation of an elementary reaction ?
AOrder is same as molecularityBOrder is less than the molecularityCOrder is grater than the molecularityDMolecularity can never be zero.
The rate and mechanism of chemical reactions are studied in chemical kinetics. The elementary reactions are single step reactions having no mechanism. The order of reaction and molecularity are same for elementary reactions. The rate of forward reaction aA+bB→cC+dD is given as :
rate = =(dxdt)=−1ad[A]dt=−1bd[B]dt=1cd[C]dt=1dd[D]dt or rate =K[A]a[B]b. In case of reversible reactions net rate expression can be written as : rate =K1[A]a[B]b–K2[C]c[D]d. At equilibrium, rate = 0. The constants K,K1,K2, are rate constants of respective reaction. In case of reactions governed by two or more steps reactions mechanism, the rate is given by the solwest step of mechanism.
For the reaction : aA→bB,
log[−dAdt]=log[dBdt]+0.6, then a:b is :
View SolutionThe rate and mechamical reaction are studied in chemical kinetics. The elementary reactions are single step reaction having no mechanism. The order of reaction and molecularity are same for elementary reactions. The rate of forward reaction aA+bB→cC+dD is given as:
rate=(dxdt)=−1ad[A]dt=−1bd[B]dt=1cd[C]dt=1dd[D]dt or expression can be written as : rate =K1[A]a[B]b−K2[C]c[D]d. At equilibrium, rate =0. The constants K,K1,K2 are rate constants of respective reaction. In case of reactions governed by two or more steps reaction mechanism, the rate is given by the slowest step of mechanism.
For a hypothetical reaction aA+bB→ Product, the rate law is: rate =K[A]x[B]y, then:
View SolutionThe rate and mechamical reaction are studied in chemical kinetics. The elementary reactions are single step reaction having no mechanism. The order of reaction and molecularity are same for elementary reactions. The rate of forward reaction aA+bB→cC+dD is given as:
rate=(dxdt)=−1ad[A]dt=−1bd[B]dt=1cd[C]dt=1dd[D]dt or expression can be written as : rate =K1[A]a[B]b−K2[C]c[D]d. At equilibrium, rate =0. The constants K,K1,K2 are rate constants of respective reaction. In case of reactions governed by two or more steps reaction mechanism, the rate is given by the slowest step of mechanism.
For the reaction, aA→bB,
log[−dAdt]=log[dBdt]+0.6, then a:b is:
View SolutionThe rate and mechamical reaction are studied in chemical kinetics. The elementary reactions are single step reaction having no mechanism. The order of reaction and molecularity are same for elementary reactions. The rate of forward reaction aA+bB→cC+dD is given as:
rate=(dxdt)=−1ad[A]dt=−1bd[B]dt=1cd[C]dt=1dd[D]dt or expression can be written as : rate =K1[A]a[B]b−K2[C]c[D]d. At equilibrium, rate =0. The constants K,K1,K2 are rate constants of respective reaction. In case of reactions governed by two or more steps reaction mechanism, the rate is given by the slowest step of mechanism.
At the point of intersection of the two curve shown for the reaction: A→nB the concentration of B is given by:
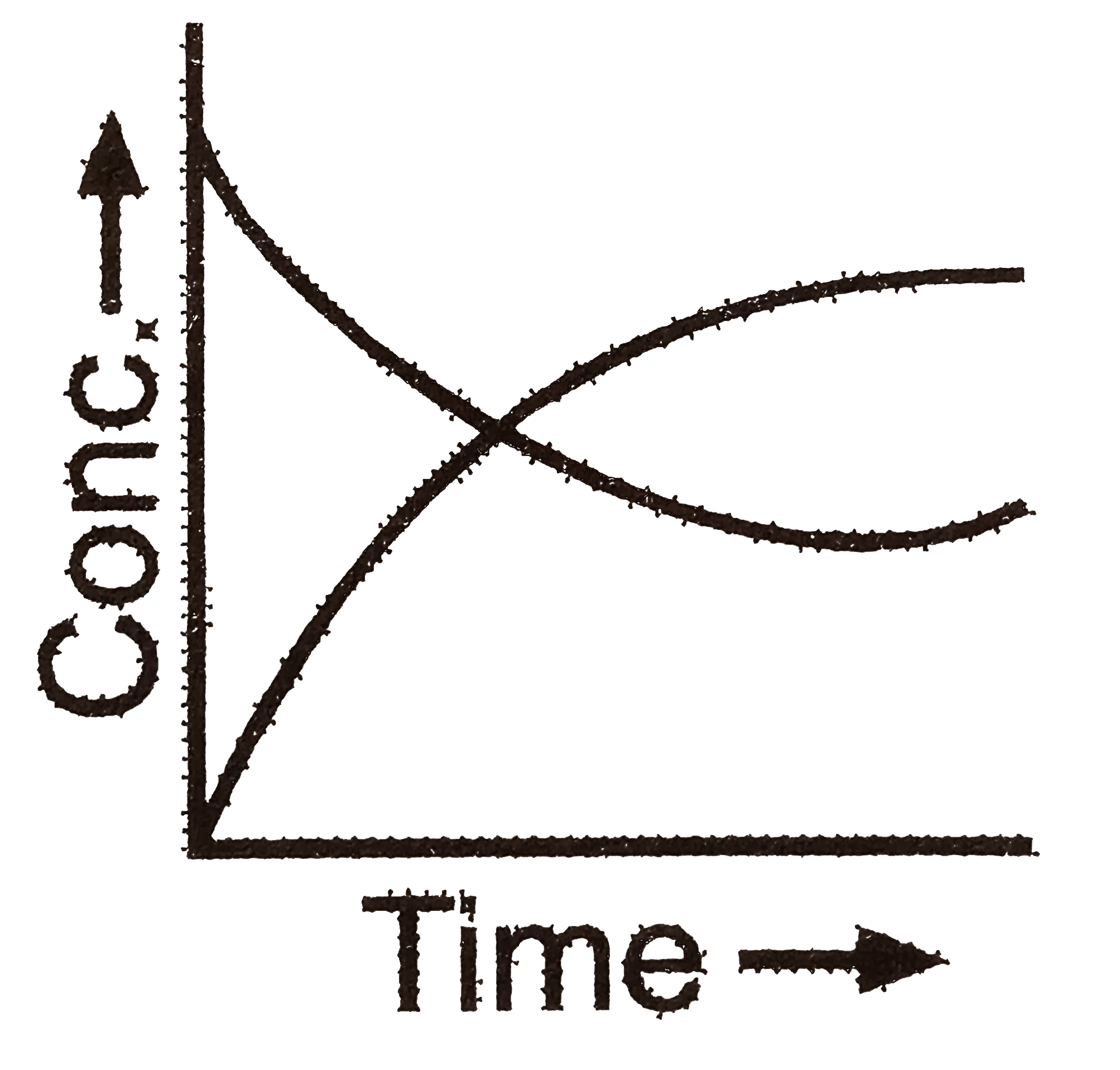
View SolutionThe rate and mechamical reaction are studied in chemical kinetics. The elementary reactions are single step reaction having no mechanism. The order of reaction and molecularity are same for elementary reactions. The rate of forward reaction aA+bB→cC+dD is given as:
rate=(dxdt)=−1ad[A]dt=−1bd[B]dt=1cd[C]dt=1dd[D]dt or expression can be written as : rate =K1[A]a[B]b−K2[C]c[D]d. At equilibrium, rate =0. The constants K,K1,K2 are rate constants of respective reaction. In case of reactions governed by two or more steps reaction mechanism, the rate is given by the slowest step of mechanism.
The rate of formation of SO3 in the following reaction,
2SO2+O2→2SO3 is 10gsec−1
The rate of disappearance of O2 will be:
View Solution

