Which one of the following is not oxidized by acidified KMnO4 ?
A
Sodium oxalate
B
Potassium iodide
C
Ferrous sulphate
D
Sodium sulphate
Video Solution
Text Solution
The correct Answer is:D
Sodium sulphite is oxidised to sodium sulphate
Na2SO3+[O]→Na2SO4(2KMnO4+3H2SO4→K2SO4+2MnSO4+3H2O+5[O])
|
Similar Questions
Knowledge Check
Which of the following is oxidised by KMnO4
AMethaneBPentaneCIsobutaneDNeopentaneWhich of the following will not decolourise acidified KMnO4 ?
AS2O2−4BS2O2−5CS2O2−3DS2O2−7Which of the following alcohol can not be oxidized by KMnO4
A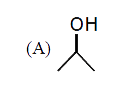 B
B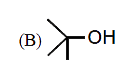 C
C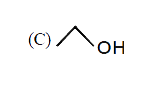 D
D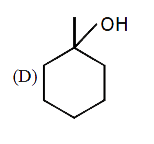
Which of the following on oxidation with hot alkaline KMnO4 gives benzoic acid?
View SolutionWhich of the following will not produce benzoic acid by oxidation with alkaline KMnO4 ?
View SolutionWhich of the following on oxidation with alkaline KMnO4 followed by acidification with HCl gives benzoic acid ?
View SolutionWhich of the following on treatment with hot concentrated acidified KMnO4 gives 2 - methylhexane -1,6 - dioic acid the only organic product ?
View SolutionWhich of the followinig alkynes will produce isobutyric acid and CO2 on oxidation with hot concentrated alkaline KMnO4?
View Solution
