The plot of partial vapour vapour pressure of solvent verses its mole fraction in the solution of a constant temp.is
a straight line
a straight line parallel to one axis
a straight line passing through origin
none of the above
The correct Answer is:C
Psolvent∝Xsolvent
Similar Questions
The vapour pressure of pure benzene and toluene at 40∘C are 184.0 torr and 59.0 torr, respectively. Calculate the partial presure of benzene and toluene, the total vapour pressure of the solution and the mole fraction of benzene in the vapour above the solution that has 0.40 mole fraction of benzene. Assume that the solution is ideal.
View SolutionIdentify the law which is stated as:
For any solution, the partial vapour pressure of each volatile component in the solution is directly proportional to its mole fraction.
View Solution
Knowledge Check
Relative lowering of vapour pressure is ____ the mole fraction of the solute.
Aequal toBequal to halfCtwiceDthree timesThe plot of total vapour pressure as a function of mole fraction of the component of an ideal solution formed by mixing liquids X and Y is
A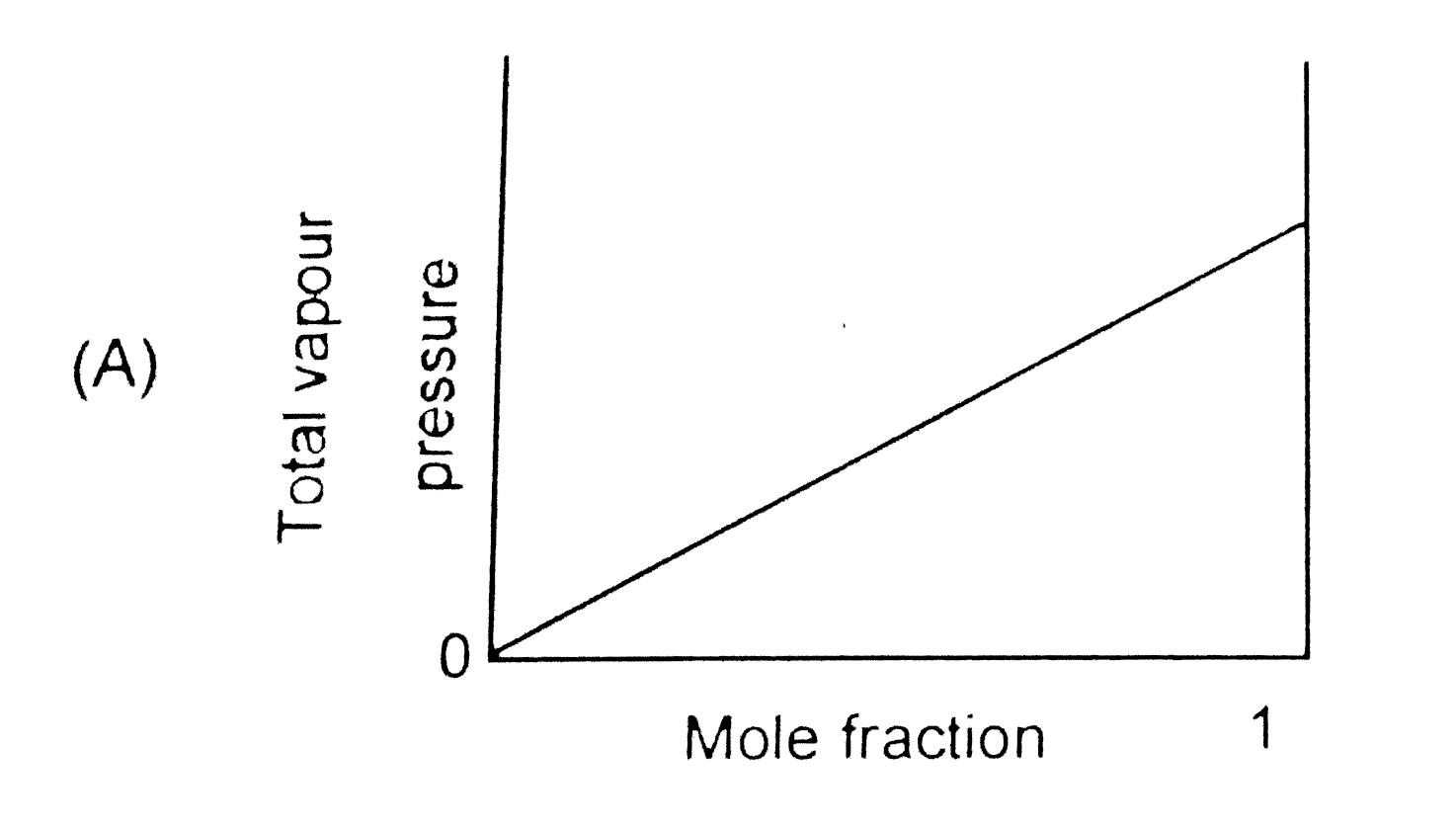 B
B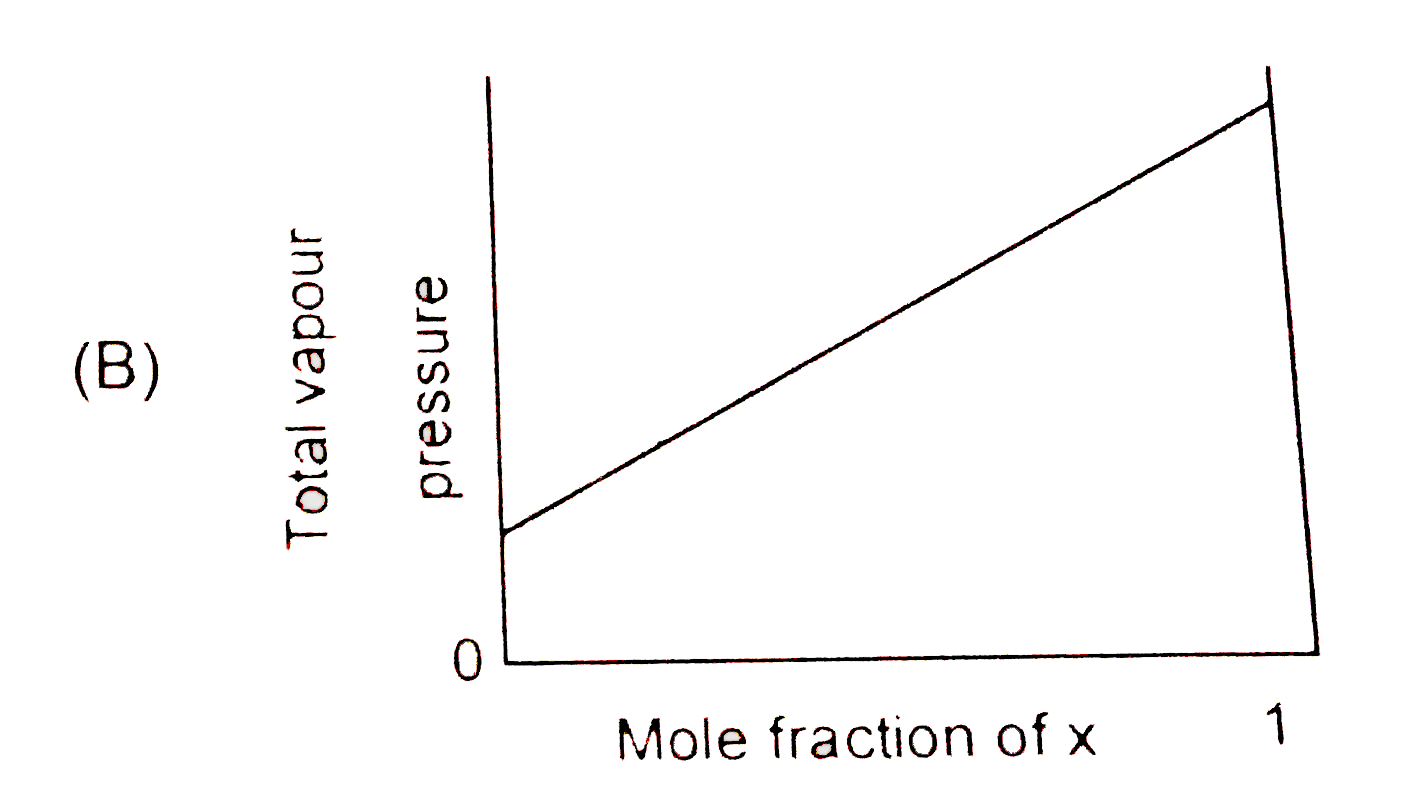 C
C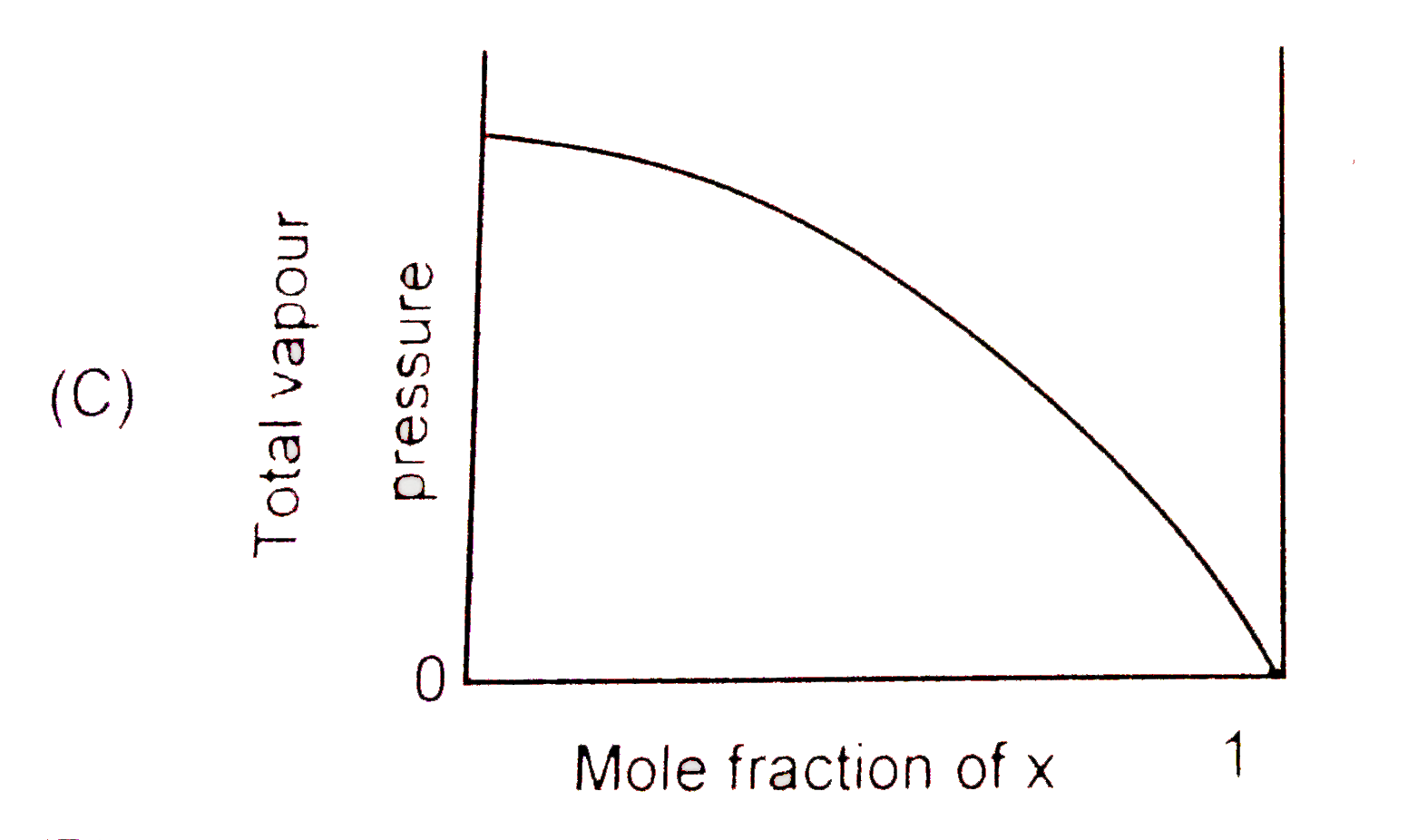 D
D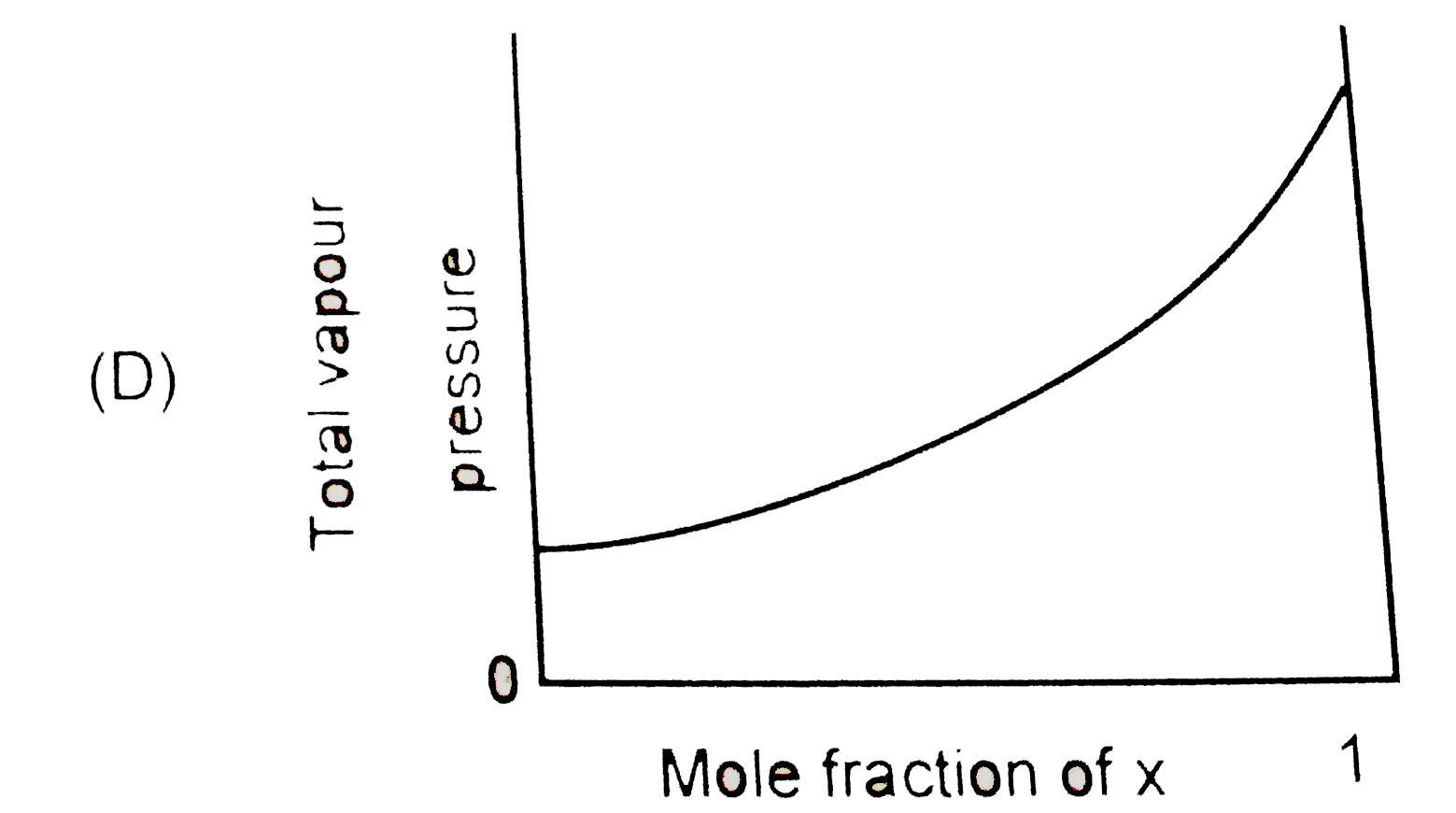
The relative lowering of vapour pressure of a solvent by the addition of a solute is :
1) proportional to the molarity of the solution
2) proportional to the molality of the solution
3) equal to the mole fraction of the solute
4) equal to the mole fraction of the solvent.Aproportional to the molarity of the solutionBproportional to the molality of the solutionCequal to the mole fraction of the soluteDequal to the mole fraction of the solvent.
If P∘ and Ps are vapour pressure of solvent and its solution, respectively, χ1 and χ2 are mole fractions of solvent and solute, respectively, then
View SolutionIf P∘ and Ps are vapour pressure of solvent and its solution, respectively, χ1 and χ2 are mole fractions of solvent and solute, respectively, then
View SolutionAssertion (A) : The vapour phase is always richer in the component which is less volatile.
Reason (R) : The relative lowering of the vapour pressure of a solution is proportional to mole fraction of the solvent.
View SolutionAddition of a non-volatile solute causes lowering in vapour pressure of a solvent from0.8atm to0.2atm . What is the mole fraction of solvent ?
View SolutionThe vapour pressure of solvent is 20 torr, while that of its dilute solution is 17 torr, the mole-fraction of the solvent is
View Solution

