Which among the following is a false statement ?
A
SO3, is obtained by the catalytic oxidation of SO2
B
SO3 has trigonal planar geometry is gaseous state
C
SO3, in gaseous state has alls Obonds equivalent
D
SO2 is basic in nature
Text Solution
The correct Answer is:D
SO2 is acidic in nature
b) Since there are three electron pairs (all bond-pairs), the orientation of three electron pairs will be trigonal planar in accordance with VSEPR theory.
c) SO3 is a hybrid of three canonical forms : 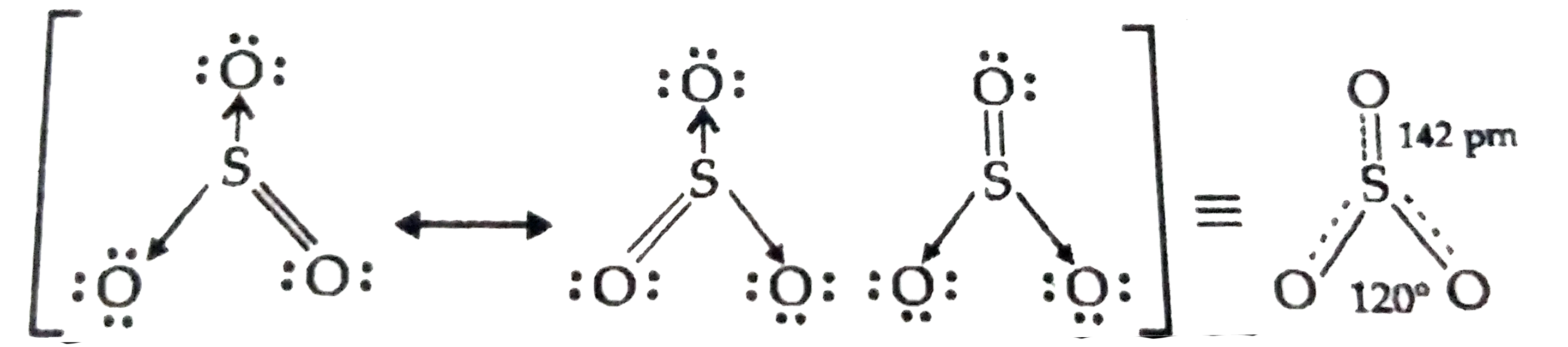
|
Similar Questions
Knowledge Check
Which among the following is the false statement ?
AWater boils below 100∘ C on mountain peaks.BIce undergoes sublimation on surface of moon.CIce melts above 0∘C on mountain peaks.DCooking of food is faster on mountain peaksAmong the following the false statement is
Aammeter is connected in series and maximum current flows through isBvoltmeter is connected in parallel and potential is maximumCammeter is connected in series and current through it is negligibleDvoltmeter is connected in parallel and current through it is negligible.If p,q and r are false statements, then which among the following is false ?
A−q∨r is trueB−r∨q is trueC(−q∨r)∧(−r∨q) is falseDp∧[(−q∨r)∧(−r∧q)] is false.

