Number of unpaired electrons in sulphur is
A
2
B
6
C
8
D
1
Video Solution
Text Solution
The correct Answer is:A
The electronic configuration of sulphur is as: S(Z=16),1s2,2s2,sp6,3s2,3p4 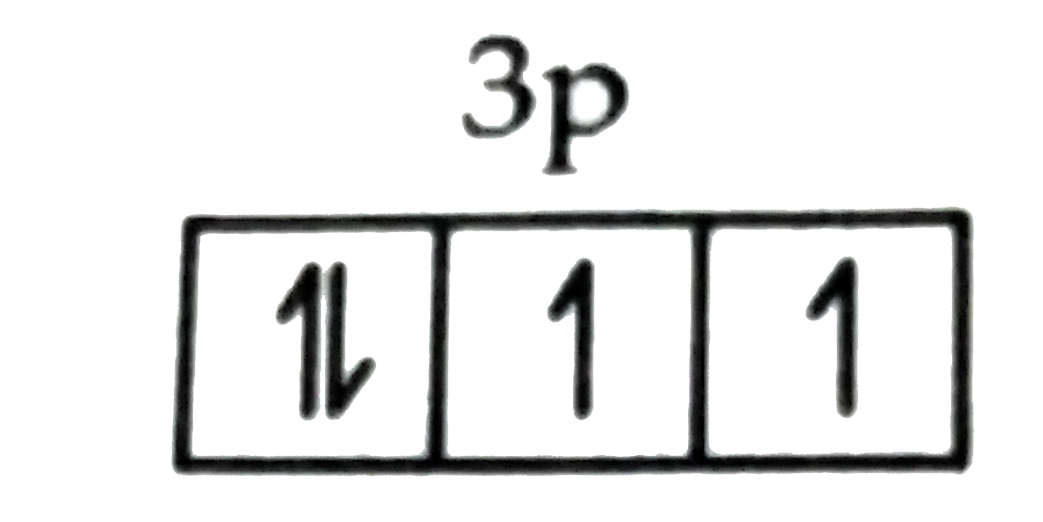
Hence, it has two unpaired electrons.
|
Similar Questions
Knowledge Check
Number of unpaired electrons on sulphur in sulphur dioxide gas is ?
A2B6C8D1Number of unpaired electrons in inert gas is:
A0B8C4D18Number of unpaired electrons in inert gas is
AZeroB8C4D18
The complex in which the number of unpaired electrons is zero is
View SolutionNumber of unpaired electrons in the ground state of beryllium atom is
View SolutionNumber of unpaired electrons in Mn3+ is
View SolutionNumber of unpaired electrons in Mn4+ is
View SolutionThe number of unpaired electrons in ferrous ion is
View Solution
