Which of the following ions is paramagnetic ?
A
Cu+
B
Zn+2
C
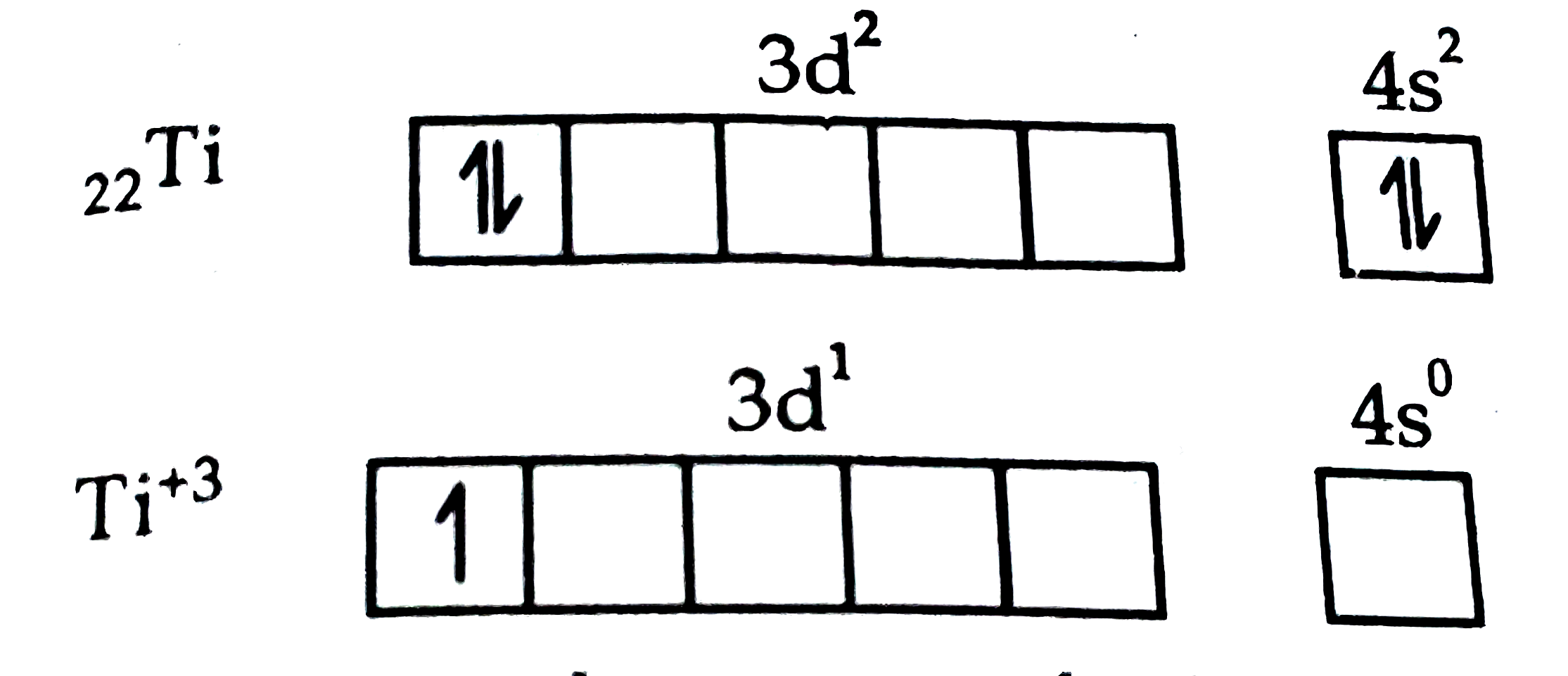
Ti+3
D
Ti+4
Video Solution
Text Solution
The correct Answer is:C
Ti3+ is paramagnetic due to the presence of an unpaired electron.
|
Similar Questions
Knowledge Check
Which of the following ion is paramagnetic
ALa3+(Z=57)BLu3+(Z=71)CYb2+(Z=70)DSm3+(Z=62)Which of the following ion is paramagnetic
ALa3+(Z=57)BLu3+(Z=71)CYb2+(Z=70)DSm3+(Z=62)Which of the following ionsis paramagnetic?
A[Ni(H2O)6]2+B[Fe(CN)6]4−C[Ni(CO)4]D[Ni(CN)4]2−
