From Ce to Lu atomic size decreases
18 Pm
8Pm
12Pm
10Pm
The correct Answer is:D
Similar Questions
Knowledge Check
Metallic Character
The ability of an atom to donate electrons and form positive ion (cation) is known as electropositivity or metallic character. Down the group, metallic character increases due to increase in atomic size and across the period, from left to right electropositivity decreases due to decrease in atomic size.
Non-Metallic Character
The ability of an atom to accept electrons to form a negative ion (anion) is called non-metallic character or electronegativity. The elements having high electro-negativity have a higher tendency to gain electrons and form anion. Down the group, electronegativity decreases due to increase in atomic size and across the period, from left to right electronegativity increases due to decrease in atomic size
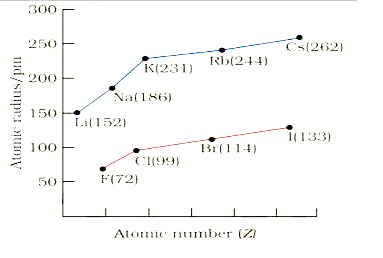
Which of the following correctly represents the decreasing order of metallic character of Alkali metals plotted in the graph?ACs>Rb>Li>Na>KBK>Rb>Li>Na>CsCCs>Rb>K>Na>LiDCs>K>Rb>Na>LiMetallic Character
The ability of an atom to donate electrons and form positive ion (cation) is known as electropositivity or metallic character. Down the group, metallic character increases due to increase in atomic size and across the period, from left to right electropositivity decreases due to decrease in atomic size.
Non-Metallic Character
The ability of an atom to accept electrons to form a negative ion (anion) is called non-metallic character or electronegativity. The elements having high electro-negativity have a higher tendency to gain electrons and form anion. Down the group, electronegativity decreases due to increase in atomic size and across the period, from left to right electronegativity increases due to decrease in atomic size
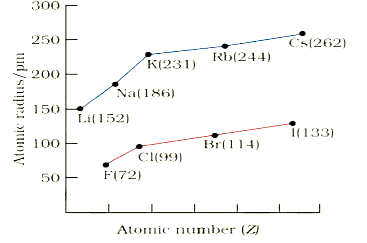
Which of the following reason correctly justifies that “Fluorine (72pm) has smaller atomic radius than Lithium (152pm)”?AF and Li are in the same group. Atomic size increases down the groupBF and Li are in the same period. Atomic size increases across the period due to increase in number of shellsCF and Li are in the same group. Atomic size decreases down the groupDF and Li are in the same period and across the period atomic size/radius decreases from left to right.Assertion:The atomic size generally increases across a period and decreases down the group.
Reason:Atomic size depends upon valence shell electronic configuration.Aa. Both Assertion and Reason are true and Reason is the correct explanation of Assertion.Bb. Both Assertion and Reason are true and Reason is not the correct explanation of Assertion.Cc. Assertion is true but Reason is false.Dd.Both Assertion and Reason are false.
Read the following and answer the question
Metallic Character:
The ability of an atom to donate electrons and form positive ion (cation) is known as electro-positivity or metallic character. Down the group, metallic character increases due to increase in atomic size and across the period, from left to right electro-positivity decreases due to decrease in atomic size
Non-Metallic Character:
The ability of an atom to accept electrons to form a negative ion (anion) is called non-metallic character or electronegativity. The elements having high electro-negativity have a higher tendency to gain electrons and form anion.
Down the group, electronegativity decreases due to increase in atomic size and across the period, from left to right electro-negativity increases due to decrease in atomic size.
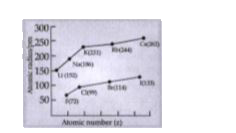
Which of the following correctly represents the decreasing order of metallic character of Alkali metals plotted in the graph ?
View SolutionAtomic size decreases in a ___________of the periodic table as the atomic number increases from left to right. (group/period)
View SolutionAssertion:The atomic size generally increases across a period and decreases down the group.
Reason:Atomic size depends upon valence shell electronic configuration.
View SolutionAssertion : La(OH)3 is more basic than Lu(OH)3 .
Reason : Basic character of hydroxides of lanthanoids decrease on moving from La3+ to Lu3+
View SolutionFrom La to Lu basicity of hydoxides
View Solution

