Chlorobenzene does not give SN1 reaction, because of
A
chlorine is attached to sp hybridized carbon atom.
B
chlorine is attached to sp3 hybridized carbon
C
resonance stabilization of benzene produces
D
self ionization of chloro benzene produces phenyl cation, which is not stabilized by resonance.
Text Solution
The correct Answer is:D
|
Similar Questions
Knowledge Check
SN1 reaction is.
Asingle step reationBa reaction involving free readical intermediateCtwo step reactionDtwo step and reaction intermediate is carbocationSN1 reaction is feasibole in-
A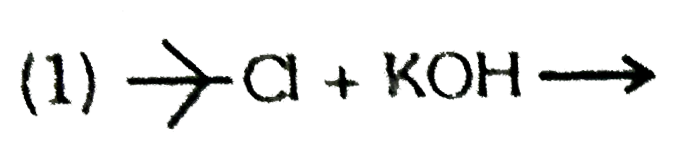 B
B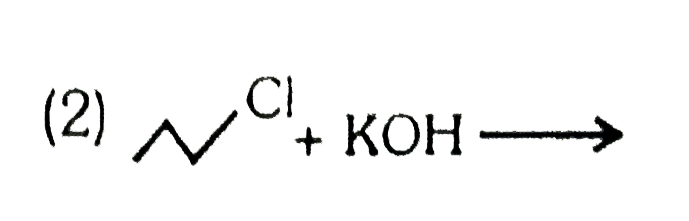 C
C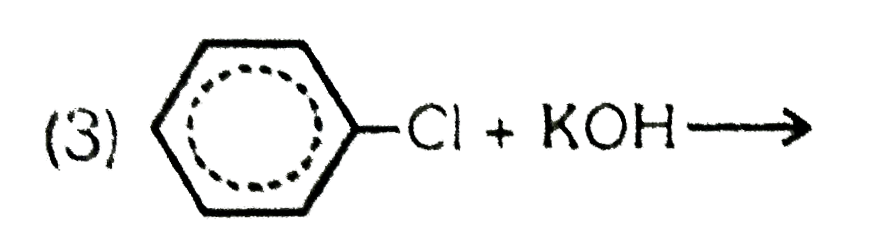 D
D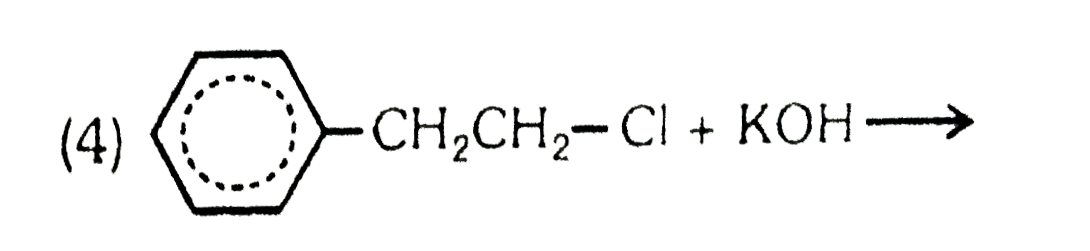
SN1 reaction is fastest in
ACH3CH2BrBCH3−CH∣Cl−CH3CCH3−CH3C∣∣CH3−ClDCH3−CH∣CH2∣CH3−Cl
The chlorine atom in chlorobenzene is ortho and para direction because
View SolutionBenzene contains double bonds but does not give addition reactions because
View SolutionChloro atom in chlorobenzene is ortho para director because
View SolutionAniline does not give coupling reactions at pH <5 because :
View SolutionS1N reaction is favoured by
View Solution

