In R '-O-R, the R' is a higher alkyl group, it is come from
alkane
alcohol
both 'a' and 'b'
not predicted
The correct Answer is:A
Similar Questions
Carbon-oxygent bond in ethers can be cleaved b halogen acids, sulphuric acid, phosphorus pentachlodie, etc. The cleavage of C-O bond take place under drastic conditions with excess of hydrogen halides.
R−O−R+2HX→2RX+H2O
If hydrogen halide is not taken in excess then a molecule of alcohol and alkyl halide are formed.
R−O−R+HI373K−−→R−OH+R−I
The order of reactivity of hydrogen halides towards the given reaction depends on the bond strength of halogenated acids, weaker is the H-X bond, greter is its reactivity. In case of unsymmetrical ethers, the site of cleavage is such that the halide is formed from the alkyl group which is smaller in size. However, when one of the alkyl group is tertiary, the halide formed is the tertiary halide.
Indiate wheterh the following statements / reactions are true or false.
In the reaction, R−O−R+HX→RX+ROH, oxonium ⎡⎢ ⎢⎣R−+O∣H−R⎤⎥ ⎥⎦ ioin is formed as intermediate.
(a) True (b) False
View SolutionCarbon-oxygent bond in ethers can be cleaved b halogen acids, sulphuric acid, phosphorus pentachlodie, etc. The cleavage of C-O bond take place under drastic conditions with excess of hydrogen halides.
R−O−R+2HX→2RX+H2O
If hydrogen halide is not taken in excess then a molecule of alcohol and alkyl halide are formed.
R−O−R+HI373K−−→R−OH+R−I
The order of reactivity of hydrogen halides towards the given reaction depends on the bond strength of halogenated acids, weaker is the H-X bond, greter is its reactivity. In case of unsymmetrical ethers, the site of cleavage is such that the halide is formed from the alkyl group which is smaller in size. However, when one of the alkyl group is tertiary, the halide formed is the tertiary halide.
Indiate wheterh the following statements / reactions are true or false.
Alkyl aryl ethers react with HI to give phenol and alkyl halide.
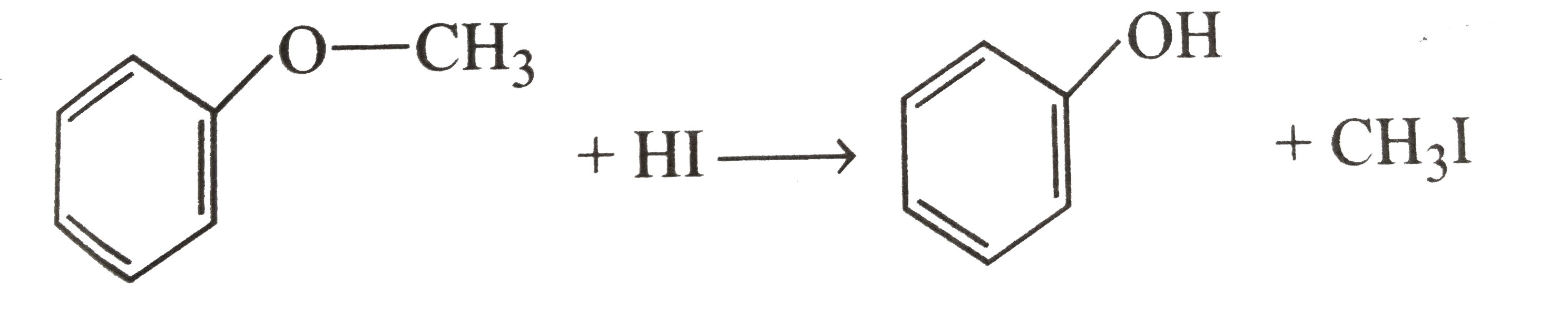
(a) True (b) False
View Solution
Knowledge Check
Following reaction is of the type
R-O-R' + HBr Cold−−→ R-Br +R' -OH
If R' is 3∘ alkyl group and R is 1∘ alkyl group , thenASN 1 with tertiary alkyl groupBSN 2 with tertiary alkyl groupCboth of the above typesDnone of the above typeswhat is the R group in glycine?
ACH2−OHBHCCH3Dnone of themKetones R−O∣∣C−R ' where R = R' alkyl group can be obtained in one step by
Aoxidation of 1∘ alcoholBhydrolysis of esterChydrolysis of acid anhydrideDoxidation of 2∘ alcohol
Give reasons for the following:
Where R is an alkyl group , R3P=O exists but R3N=O does not .
View SolutionGive reasons for the following :
(i) Where R is an alkyl group, R3P=O exists but R3N=O does not.
(ii) PbCl4 is more covalent than PbCl2.
(iii) At room temperature, N2 is much less reactive.
View Solutionketones [R−C||O−R1 , where R=R1 = alkyl groups ] can be obtained in one step by
View SolutionKetones (R1COR2),R1=R2 -alkyl group, can be obtained in one step by
View SolutionAssertion : When the alkyl group is small like −CH3 group, there is n steric hindrance to H-bonding.
Reason (R) : In aniline or other arylamines, the −NH2 group is attached directly to the benzene ring.
View Solution

