Molecular formula(A)C4H10O on acid hydrolysis gives two mole of same alcohol.The compound A is
2-methoxy propane
1-methoxy propane
t-butyl alcohol
Diethyl ether
The correct Answer is:D
Similar Questions
Knowledge Check
Molecular formula C4H10O exhibits
Achain isomerismBposition isomerismCmetamerismDall of theseMolecular formula C3H6O2 on acid hydrolysis gives A and B. The compound 'A' reduce Tollens reagent. The possible structure of C3H6O2 will be
ACH3−CH2−COOHBCH3−COOCH3CH−COOC2H5DCH3−O∣∣C−CH3Molecular formula C4H8O2 on acid hydrolysis gives acetic acid and ethanol. The structures of C4H8O2 will be
A B
B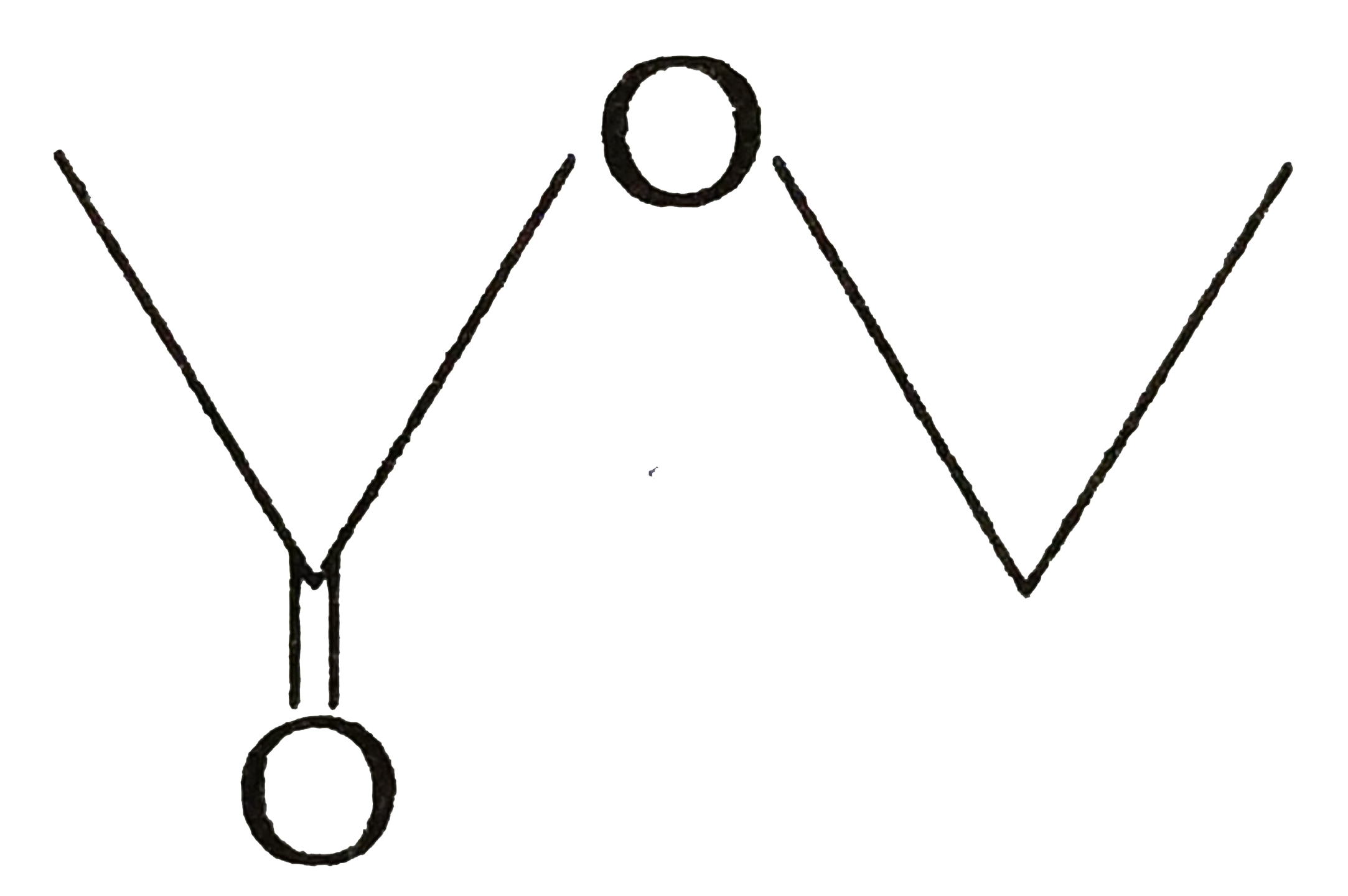 C
C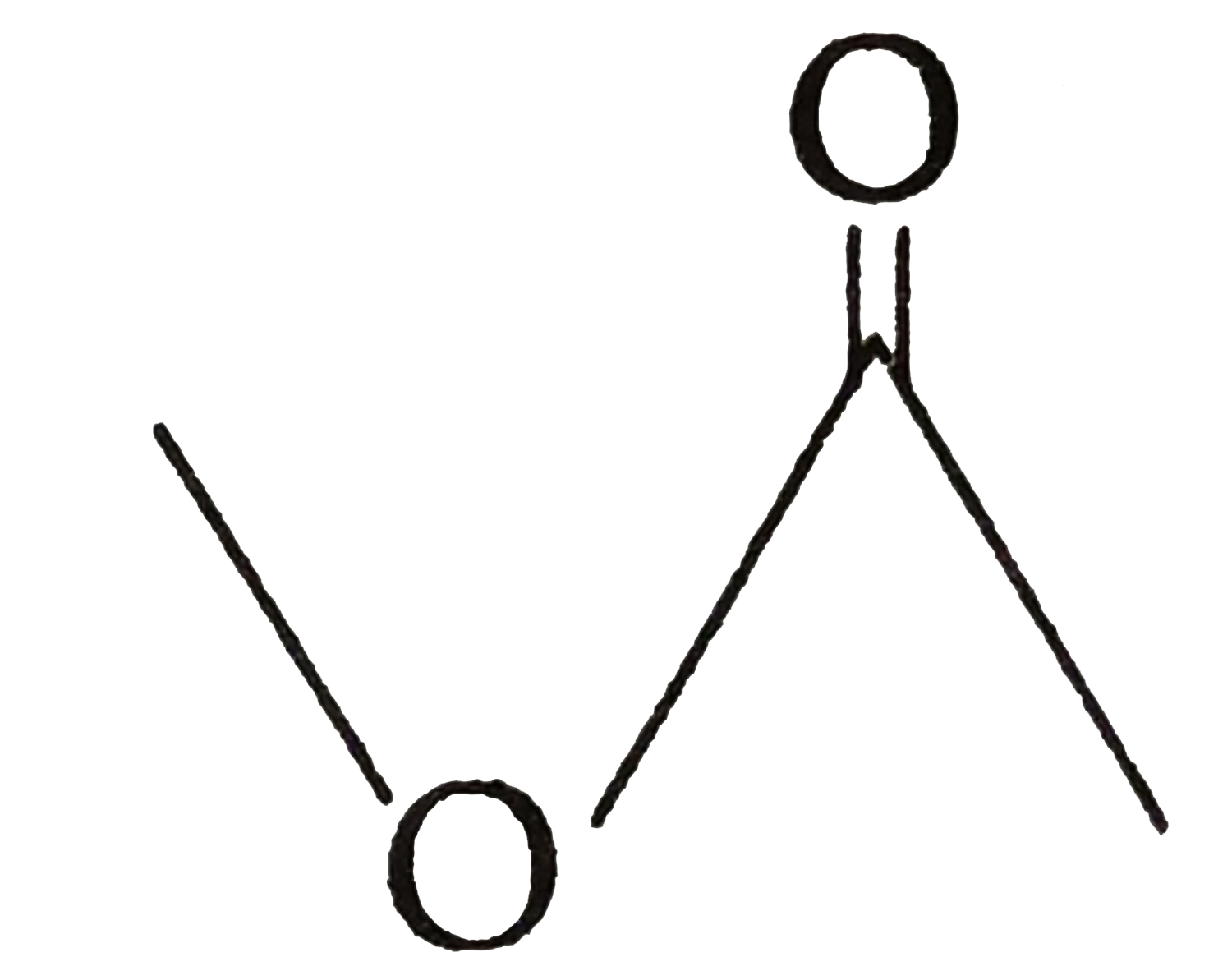 D
D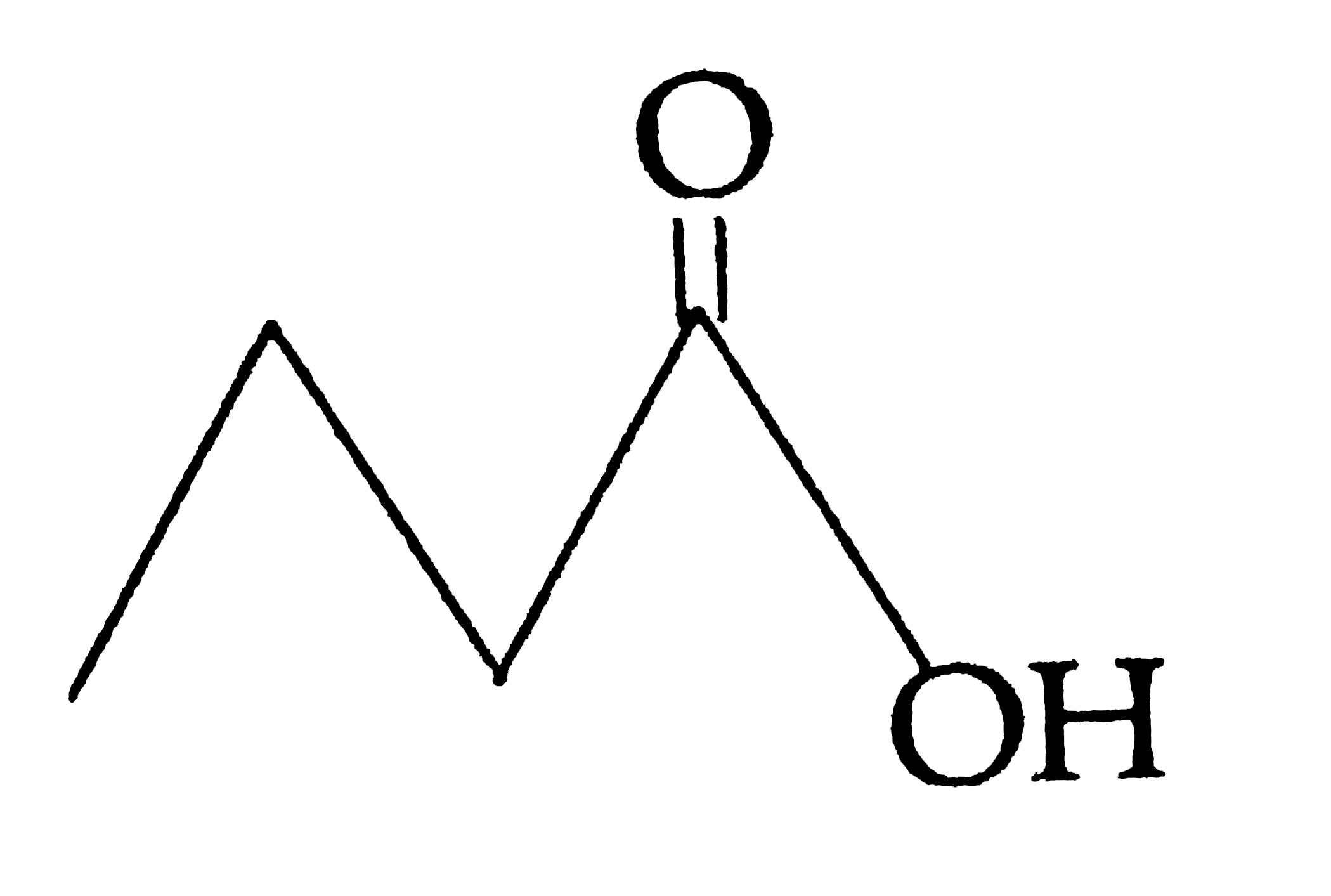
Compound (X) having molecular formula C6H12Cl2 on hydrolysis gives a ketone. Therefore (X) will be
View SolutionA compound containing molecular formula C5H10Cl2 on hydrolysis gives compound containing molecular formula C5H10O, which reacts with NH2OH and also forms iodoform but does not give Fehling test original compound is
View SolutionA compound with molecular formula C4H10O3 is converted by the action of acetyl chloride to a compound with molecular weight 190. The original compound has :
View SolutionA compound with molecular formula C4H10O3 is converted by the action of acetyl chloride to a compound with molecular weight 190. The original compound has :
View SolutionA compound with molecular formula C4H10O4 on acylation with acetic anhydride gives a compound with molecular formula C12H18O8. How many hydroxyl groups are present in the compound?
View Solution

