But - 1- yne on hydration in the presence of 40%H2SO4 and 10%HgSO4 gives




The correct Answer is:B
Similar Questions
896 mL of a hydrocarbon 'A' having carbon 87.80 % and hydrogen 12.19% weighs 3.28 g at STP. Hydrogenation of 'A' gives 2-methylpentane. Also 'A' on hydration in the presence of H2SO4 and HgSO4 gives a ketone 'B' having molecular formula C6H12O. The ketone 'B' gives a positive iodoform test. Find the structure of 'A' and give the reaction involved.
View SolutionAddition of water to ethyne in the presence of H2SO4 and HgSO_4 gives
View Solution
Knowledge Check
Hydration of
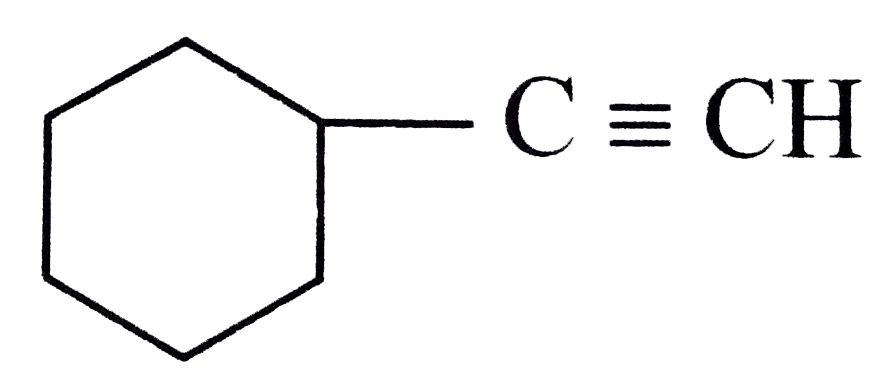 in presence of H2SO4/HgSO4 gives ( as a major product ) A
in presence of H2SO4/HgSO4 gives ( as a major product ) A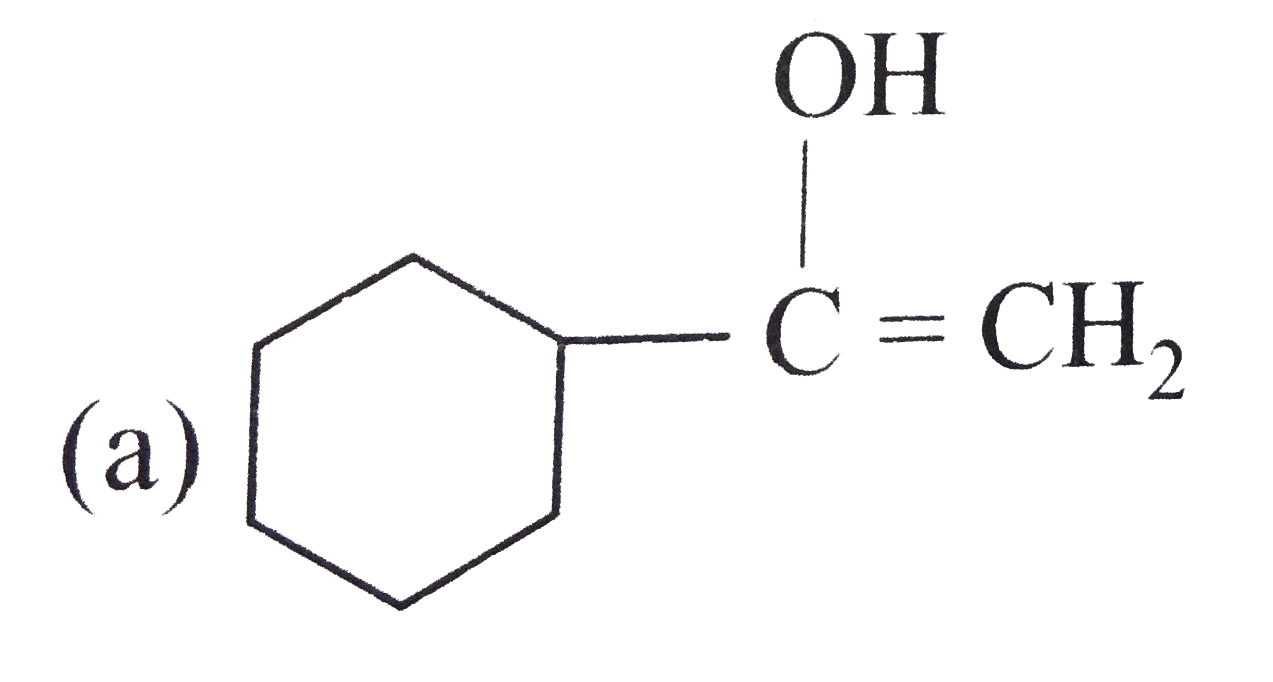 B
B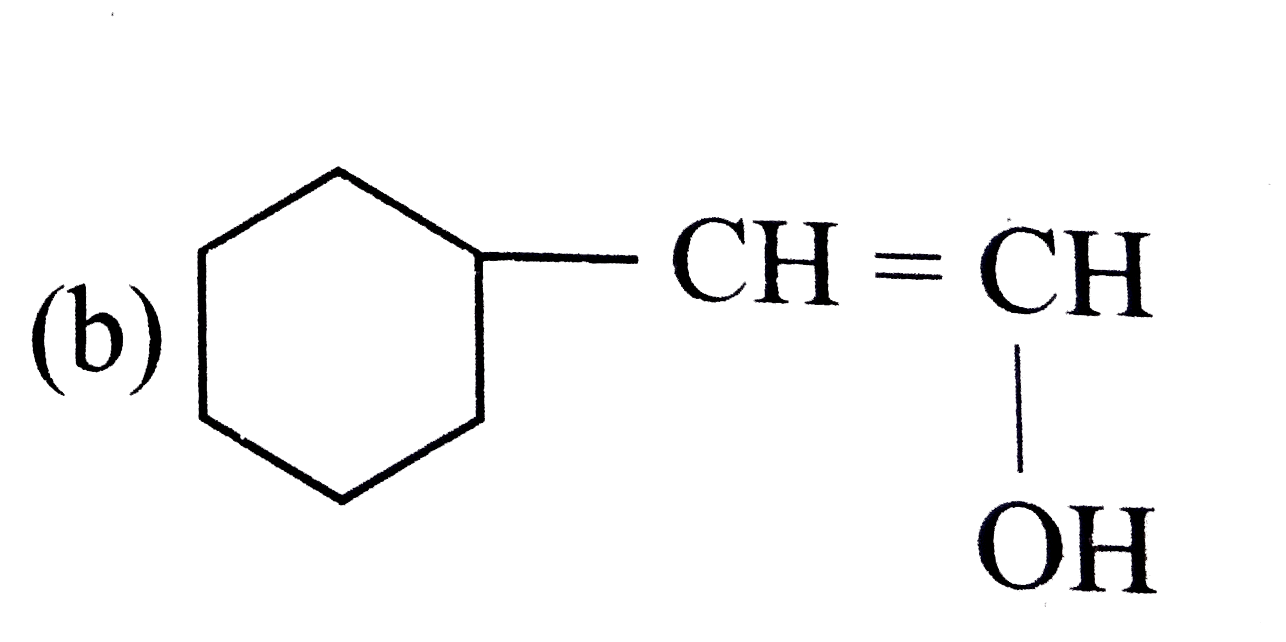 C
C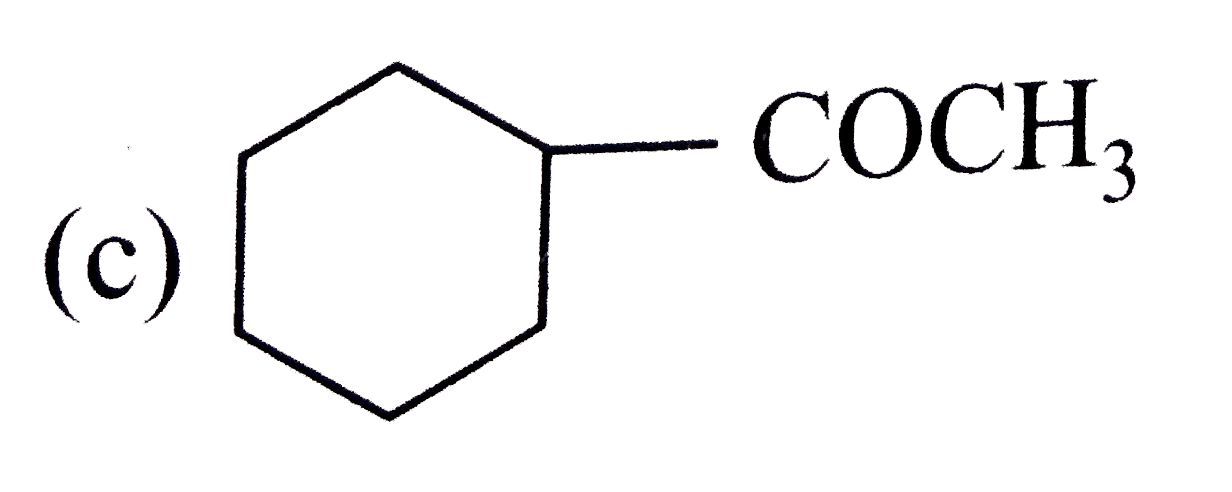 D
D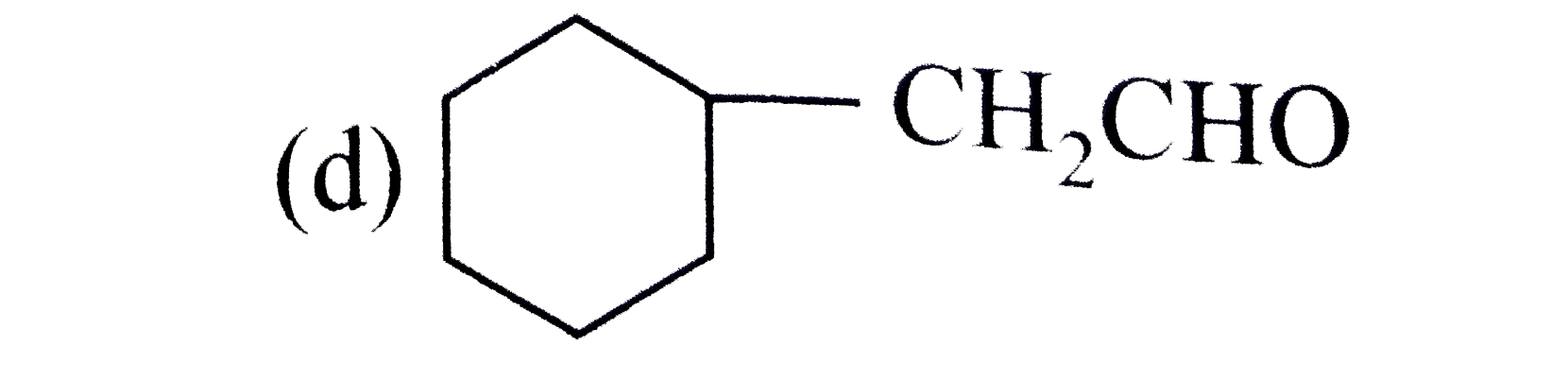
The intermediate compound formed when acetylene s hydrated in presence of dil H2SO4 and HgSO4 is
AAcetaldehydeBEthenolCVinyl chlorideDEthenalAddition of water to ethyne in the presence of H2SO4 and HgSO_4 gives
AAcetoneBAcetaldehydeCFormaldehydeDGlyoxal
896 mL vapour of a hydrocarbon 'A' having carbon 87.80% and hydrogen 12.19% weighs 3.28 g at STP. Hydrogenation of 'A' gives 2-methylpentane, Also'A' on hydration in the presence of H2SO4 and HgSO4 gives a ketone 'B' gives a positive iodoform test. Find the structure of 'A'.
View SolutionAssertion : Acetaldehyde can be prepared by addition of water to ethyne in the presence of H2SO4andHgSO4.
Reason : Higher alkynes give higher aldehydes.
View SolutionAssertion : Acetaldehyde can be prepared by addition of water to ethyne in the presence of H2SO4andHgSO4.
Reason : Higher alkynes give higher aldehydes.
View SolutionAcetylene reacts with 42% H2SO4 containing 1% HgSO4 to give:
View SolutionAcetylene on treatment with dil. H2SO4 having HgSO4 gives:
View Solution

