Carboxylic acid is isomeric with
A
aldehydes
B
alcohols
C
esters
D
saturated ketones
Text Solution
The correct Answer is:C
|
Similar Questions
Knowledge Check
Carboxylic acid can
Adecompose carbonate and evolve CO2Breacts with metal forming H2 gasCneutralise ammonium hydroxide form saltDall of the above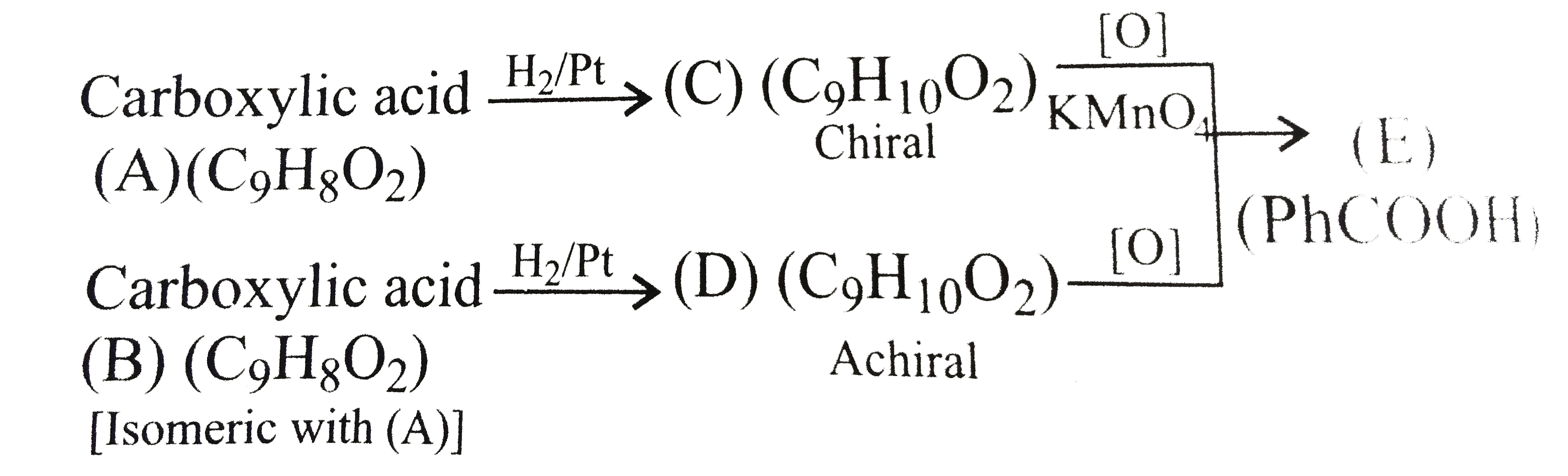
Carboxylic acid (A) is:A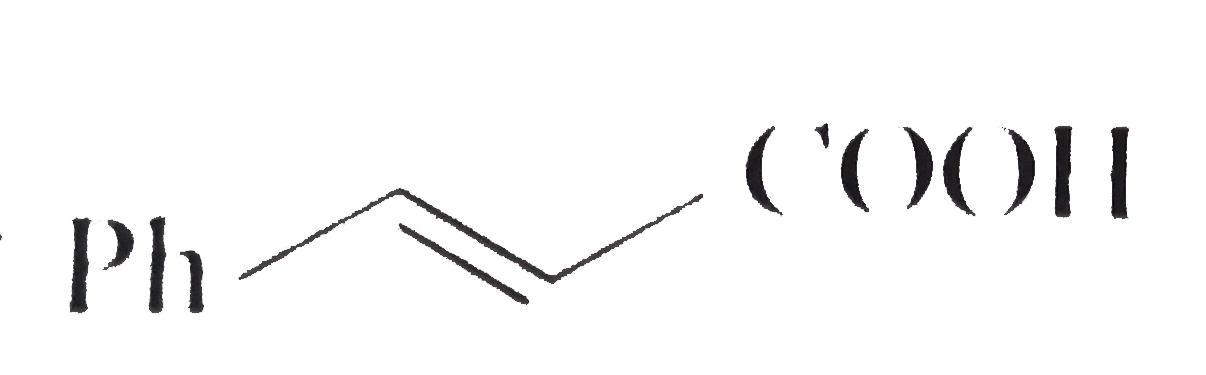 B
B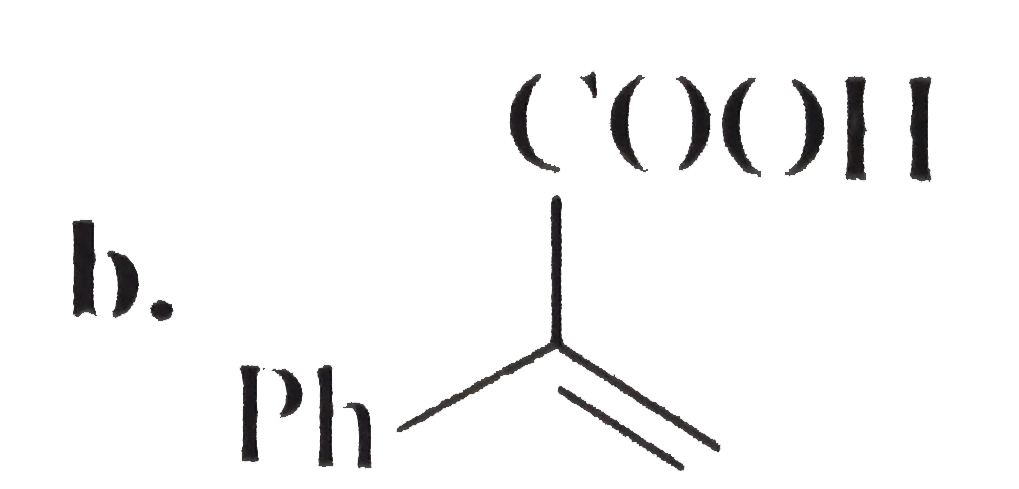 C
C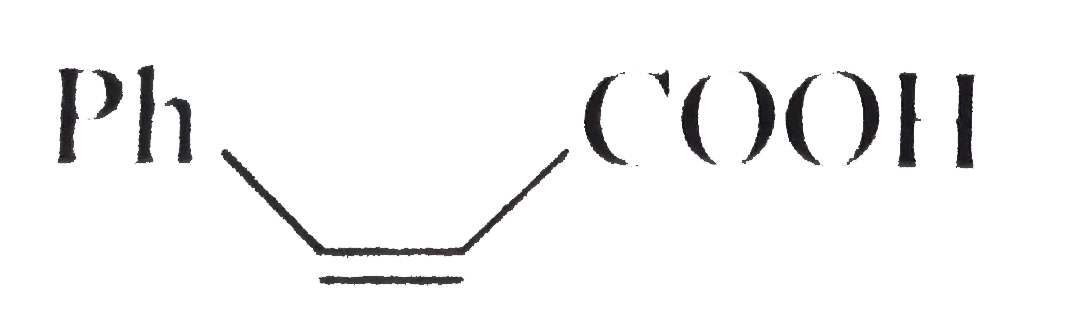 DBoth (a) and (c )
DBoth (a) and (c )Carboxylic acids dimerise due to
Ahigh molecular weightBcoordinate bondingCintermolecular hydrogen bondingDcovalent bonding.
Carboxylic acids dissociate in water to give _______ stabilised carboxylate anions and hydronium ion.
View SolutionIntroduction OF Carboxylic acid || Naming OF Carboxylic acids || Isomersm || General Properties OF Carboxylic acids ||Acidity OF Carboxylic acids || General Method OF Prepartion OF Carboxylic acids from Various Compounds
View SolutionCarboxylic acids dimerise due to
View SolutionAssertion (A) : Higher carboxylic acids are practically insoluble in water.
Reason (R) : Carboxylic acids dissociate in water to give resonance stabilised carboxylic anions and hydronium ion.
View SolutionThe acidic nature of carboxylic acid is due to
View Solution

