If hydrolysis of ester is carried out inpresence of an acid , usig water containing redioactive oxygen atom, the product most likely to be radioactive is ,
alcohol
ester
acid
both 'a' and 'c'
The correct Answer is:C
RCOOR'+H2Oconc.H2SO4⇐=====⇒ΔRCOOH+R'OH
The radioactive oxygen must be in acid. This technique is known as tracer technique.
Similar Questions
Why iodination of alkanes is carried out in the presence of oxidizing agents?
View SolutionNatural radioactivity is a spontaneous and self disruptive activity exhibated by a number of heavy elements in nature. Thus a heavy element disintegrates by itself without being forced by any external agent to do so. According to radioactive decay law, the number of atoms disintegrated per second (i.e., rate of disintegration of radioactive atoms) at any instant is directly propotional to the number of radioactive atoms actually in the sample at that instant, i.e., −dNdt∝NorR=−dNdt=λN, where λ is decay constant. Read the above passage and answer the following questions:
(i) The count rate form a radioactive sample containing 1016 atoms is 4×106 per second. What is the value of decay constant?
(ii) Name the three types of radiactive radiations. Which one of them is most penetrating?
(iii) What does radioactive decay law imply in day to day life?
View Solution
Knowledge Check
The hydrolysis of an ester was carried out separately with 0.1 M HCl and 0.1 M H2SO4. Which of the following will be true?
AkHCl>kH2SO4BkHCl<kH2SO4CkHCl=kH2SO4DunpredictableWhen beet root cylinders are washed and then placed in cold water, anthocyanin does not come out. This indicates that most likely the plasme membrane is
Apereable to anthocyaninBImpermeable to anthocyaninCDifferentially permeable to anthocyanin.DDead structureIf acetic acid reacts with methyl alcohol containing labelled oxygen atom . Inpresence of dry HCl, the labelled oxygen atom, at the complete reaction will be found in
Amethyl acetateBwaterCmay be 'a' and 'b'Dwater
The pH scale provides a convenient way to express the acidity and basicity of dilute aqueous solution. It depends upon the degree of dissociation of acids and bases in water Ionic product of water in the product of molar concentration of H+ And OH− ions at equilibrium at a given temperature. At 25∘C, Kw is 10×10−14. The salts of weak bases and strong acids or weak acids and strong bases also give acidic or basic solution due to hydrolysis.
What happens to ionic product of water at 27∘ C if some acid is added to it?
View SolutionAn oxyacid contains three hydrogen atoms, one phosphorus atom and four oxygen atoms.
Name the acid.
View SolutionIf acetic acid reacts with methyl alcohol containing labelled oxygen atom in presence of dry HCl.The labeled oxygen atom, at the complete reaction will be found in
View SolutionAmides undergo hydrolysis to yield carboxylic acid plus amine on heating in either aqueous acid or aqueous base. The conditions required for amide hydrolysis are more severe than those required for the hydrolysis of esters, anhydrides or acid chlorides, but the mechanism is similar (nucleophilic acyl substitution). Nucleophilic acyl substitutions involve a tetrahedral intermediate, hence these are quite different from alkyl substitution
(RCH2BrNaCN−−−→RCH2CN)
which involves a pentavalent intermediate or transition state. One of the important reactions of esters is their reaction with two equivalent of Grignard reagent to give tertiary alcohols.
When
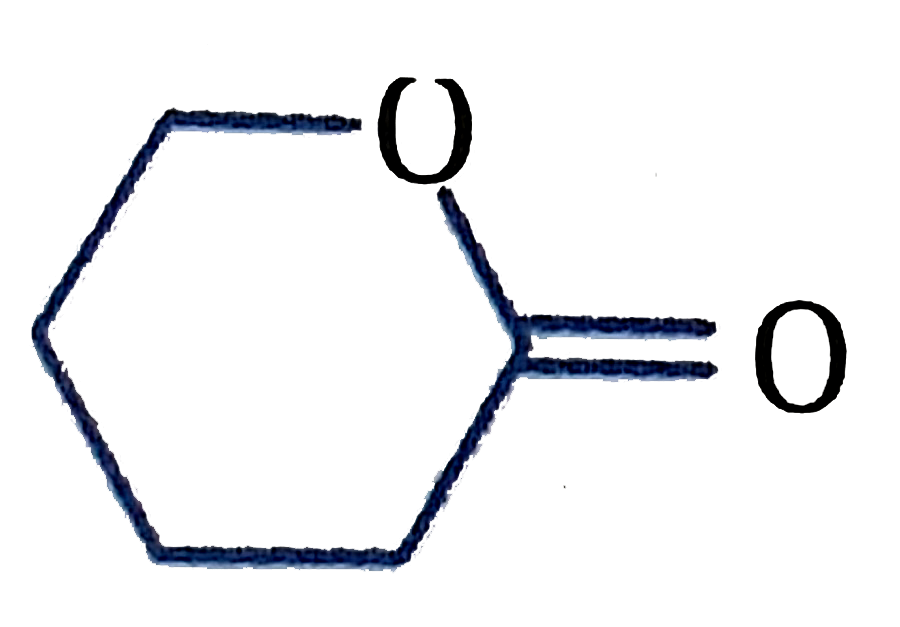
is treated with two equivalent of methyl magnesium iodide and the product acidified the final product will be
View SolutionAssertion (A): Although the concentration of ozone is at most 12 parts per million (ppm), it is very effective at absorbing the harmful ultraviolet (UV) rays of the sun and protecting life on Earth.
Reason (R) : Ozone is a molecule made of three oxygen atoms. The oxygen molecule we need to breathe contains two oxygen atoms.
View Solution

