A compound 'A' having the molecular formula C5H12O, on oxidation gives a compound 'B' with molecular formula C5H10O. Compound 'B' gave a 2, 4- dinitrophenylhydrazine derivative but did not answer haloform test or silver mirror test. The structure of compound 'A' is
A
CH3−CH2−CH2−CH2−CH2−OH
B
CH3−CH2−CH2−CH∣OH−CH3
C
CH3−CH2−CH∣OH−CH2−CH3
D
CH3−CH2−CH∣CH3−CH2−OH
Text Solution
The correct Answer is:C
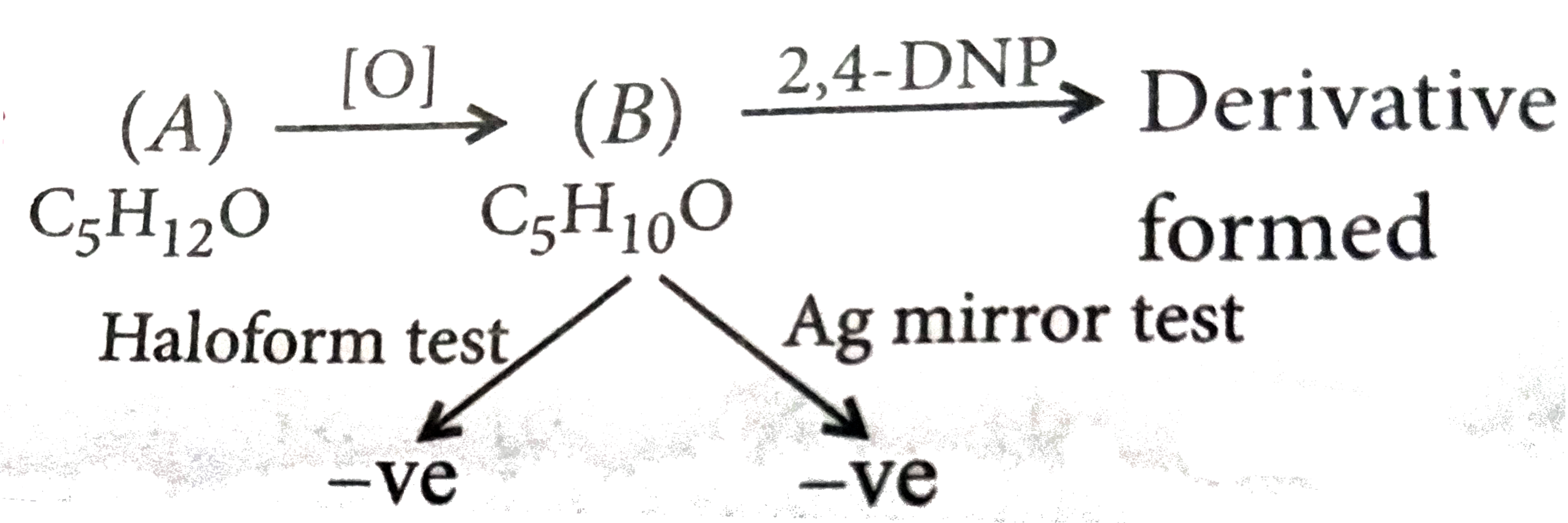
Since (B) on reaction with 2, 4-DNP forms a derivative, it implies that (B) has 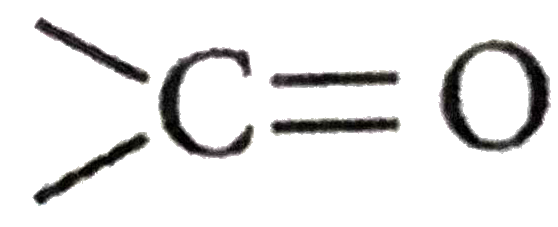 group.
group.
(B) gives -ve Tollens' test, hence it is not an aldehyde, but it is a ketone.
(B) gives -ve haloform test, thus it is not a methyl ketone.
(B) is formed from the oxidation of (A), thus (A) is a 2∘ alcohol, and among the given options,
(A) is : CH3−CH2−CH∣OH−CH2−CH3
and ∴(B) is CH3−CH2−C||O−CH2−CH3
|
Topper's Solved these Questions
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:NCERT FINGERTIPSChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:MCQs( CHEMICAL REACTIONS )
Explore 2 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:NCERT FINGERTIPSChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:MCQs(CHEMICAL REACTIONS )
Explore 11 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:NCERT FINGERTIPSChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:Physical Properties
Explore 4 Videos
- ALCOHOLS,PHENOLS AND ETHERSBook:NCERT FINGERTIPSChapter:ALCOHOLS,PHENOLS AND ETHERSExercise:Assertion And Reason
Explore 14 Videos
- AMINES Book:NCERT FINGERTIPSChapter:AMINES Exercise:NCERT EXEMPLER PROBLEMS
Explore 1 Video
Similar Questions
Knowledge Check
A compound 'A' having the molecualr formula C5H12O on oxidation gives a compound 'B' with molecular formula C5H10O compound B gave a 4-dinitrophenyl hydrazine derivative but did not answer halo form test or silver nitrate test. The structure of compound A is
ACH3CH2CH2CH2CH2OHBCH3CH2−CH2−CH| OH−CH3CCH3CH2−CH| OH−CH2−CH3DCH3CH2−CH| CH3−CH2−OHA compound having molecular formula C4H10O can show
AMetamerismBFunctional isomerismCChain isomerismDPosition isomerismCompound having molecular formula C5H12O cannot show
ATautomerismBPosition isomerismCMetamerismDFunctional isomerism
A compound X with molecular formula C3H8O can be oxidised to a compound Y with the molecular formula C2H4O2. The compound X is likely to be
View SolutionA compound X with molecular formula C3H8O can be oxidised to a compound Y with the moleculr formula C3H6O2, X is most likely to be -
View SolutionA compound (X) with the molecular formula C3H8O can be oxidized to another (Y) whose molecular formula is C6H6O2
The compound (X) may be
View SolutionA compound (A) with molecular formula C5H10 gives one monochlorination product. Compound (A) is:
View SolutionThe compound represented by the molecular formula, C7H8O are-
View Solution

