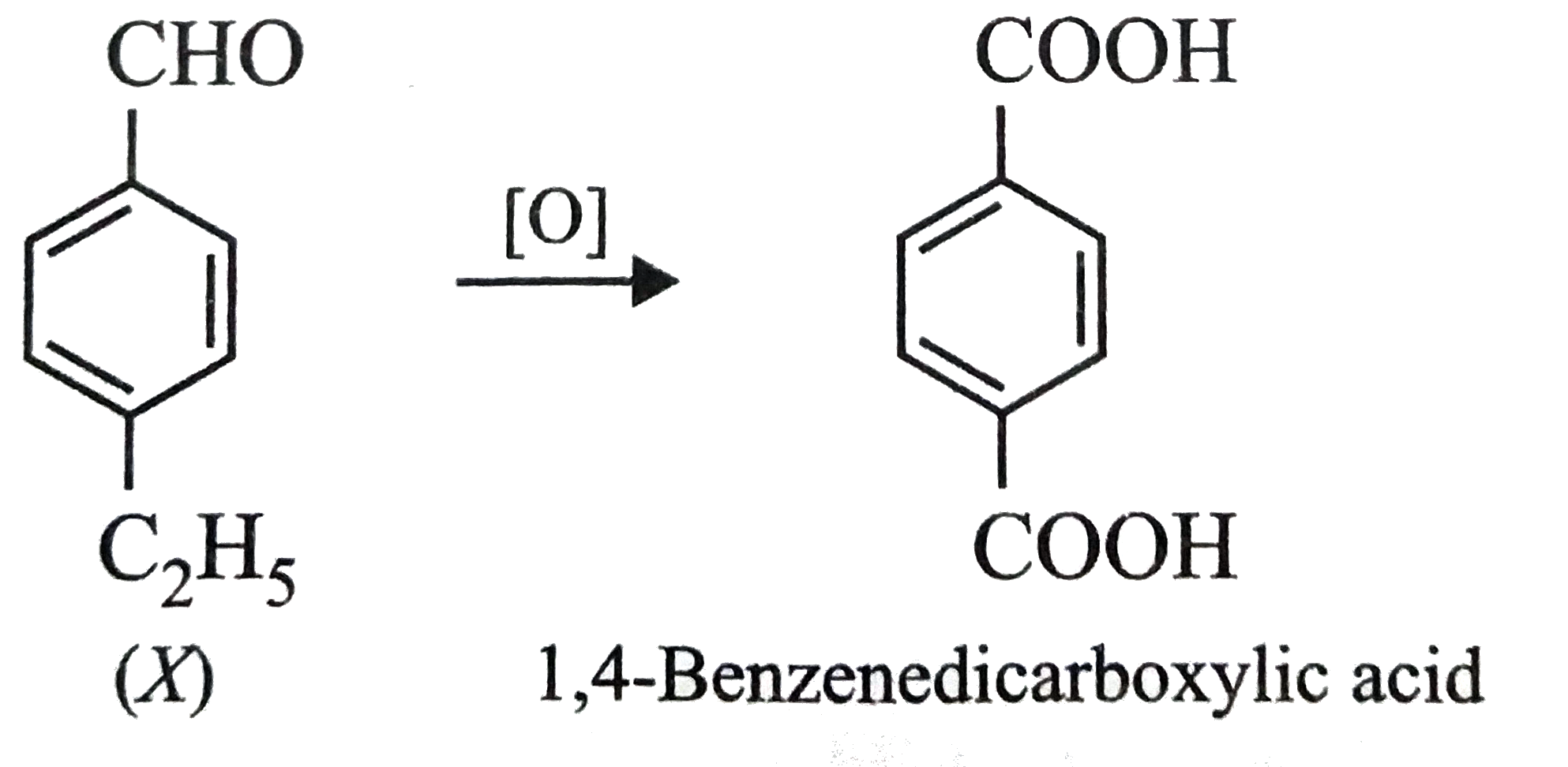
An organic compound (X) with molecular formula C9H10O gives positive 2, 4-DNP and Tollens'tests. It undergoes Cannizzaro reaction and on vigorous oxidation it gives 1, 4-benzenedicarboxylic acid. Compound (X) is
Benzaldehyde
o-methylbenzaldehyde
p-ethylbenzaldehyde
2, 2-dimethylhexanal.
The correct Answer is:C
Topper's Solved these Questions
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:NCERT FINGERTIPSChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:MCQs( CHEMICAL REACTIONS )
Explore 2 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:NCERT FINGERTIPSChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:MCQs(CHEMICAL REACTIONS )
Explore 11 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:NCERT FINGERTIPSChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:Physical Properties
Explore 4 Videos
- ALCOHOLS,PHENOLS AND ETHERSBook:NCERT FINGERTIPSChapter:ALCOHOLS,PHENOLS AND ETHERSExercise:Assertion And Reason
Explore 14 Videos
- AMINES Book:NCERT FINGERTIPSChapter:AMINES Exercise:NCERT EXEMPLER PROBLEMS
Explore 1 Video
Similar Questions
Study the given reaction and identify the process which is carried out.

View SolutionIdentify (X), (Y) andc (Z) in the given reaction :
CH3CHOK2Cr2O7−−−−−→H2SO4(X)PCl5−−→(Y)C6H6−−−−−→anh.AlCl3(Z)
View SolutionThere is a large difference in the boiling points of butanal and butan - 1 - ol due to
View SolutionAssertion : Catalytic reduction of butanal gives butanol.
Reason: Aldehydes on reduction give corresponding primary alcohols.
View SolutionThe correct increasing order of basic stregth for the following compounds is ________________ .
View SolutionAn organic compound with the molecular folmula C_(9)H_(10)O form 2,4-DNP derivative, reduces Tollens reagent, and undergoes Cannizaro reaction. On vigorous oxidation, it gives 1,2-benzenedicarboxylic acid. Identify the compound.
View SolutionAn organic commpound with the jmolecular formula C9H10O forms a 2,4-DNP derivative, reduces tollen's reagent and undergoes cannizaro reaction, on vigorous oxidation it gives 1,2-benzenedicarboxylic acid. Structure of organic compound is:
View SolutionAn aromatic compound X with molecular formula C9H10O gives following chemical tests:
(i) Form 2,4-DNP derivative
(ii) Reduces Tollen's reagent
(iii) undergoes Cannizzaro's reaction
(iv) On vigorous oxidation 1, 2-Benzene dicarboxylic acid is obtained
The compound X is ,
View SolutionAn organic compound with the molecular folmula C9H10O form 2,4-DNP derivative, reduces Tollens reagent, and undergoes Cannizaro reaction. On vigorous oxidation, it gives 1,2-benzenedicarboxylic acid. Identify the compound.
View SolutionAn organic compound with the molecular formula C_(8)H_(8)O forms 2,4-DNP derivative, reduces Tollens' reagent and undergoes Cannizzaro reaction. On vigorous oxidation, it gives 1,2-benzandicarboxylic acid. The organic compound is
View SolutionAn organic compound with the molecular formula C_(9)H_(10)O forms 2,4-DNP derivative, reduces Tollens’ reagent and undergoes Cannizzaro reaction. On vigorous oxidation, it gives 1,2-benzenedicarboxylic acid. Identify the compound.
View SolutionAn organic compound with molecular formula C9H10O forms 2,4,-DNP derivative reduces Tollen's reagent and undergoes Cannizzaro reaction .On vigorous oxidation it gives 1,4-benzene dicarboxylic acid Identify the compound .
View SolutionAn organic compound (X) with molecular formula C9H10O gives positive 2, 4-DNP and Tollens'tests. It undergoes Cannizzaro reaction and on vigorous oxidation it gives 1, 4-benzenedicarboxylic acid. Compound (X) is
View SolutionAn organic compound with the molecular formula C_(9)H_(10)O forms 2, 4 - DNP derivative, reduces Tollens' reagent and undergoes Cannizzaro reaction. On vigorous oxidation, it gives 1, 2 - benzenedicarboxylic acid. Identify the compound.
View SolutionAn organic compound with undergoes formula C_(9) H_(10) O forms 2,4-DNP derivattive, reduces Tollen's reagent ad undergoes Cannizzaro's reaction. On vigorous oxidation it gives 1,2-benzendicarboxylic acid. Identify the compound.
View Solution