Write chemical equation involved in this experiment.
Ethanoic acid reacts with ethanol in the presence of an acid catalyst and ester, ethyl ethanoate is formed. CH3COOHEthanoic acid+CH3CH2Ethanol−OHAcid−−−−→CatalystCH3(ethyl ethanoate)−COO−CH2−CH2+CH2+H2OWater
Similar Questions
Look at the figure given below and answer the following questions
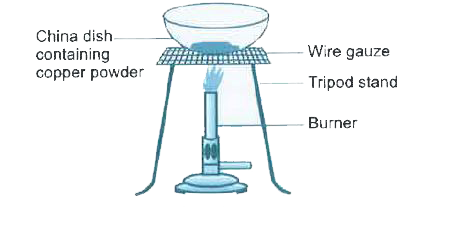
Write the chemical equation involved in this process.
View SolutionWrite the chemical equation involved in the preparation of XeF4.
View SolutionWrite the chemical equations involved in the preparation of elemental boron from mineral colemanite.
View SolutionWrite the chemical equations involved in the preparation of elemental boron from mineral colemanite.
View SolutionWrite the chemical equation involved in the detection of nitrogen by Lassaigne's test.
View SolutionWrite the chemical equations involved in the preparation of the following:
(a) XeF4 (b) H3PO3
View SolutionWrite the chemical equations involved in the extraction of lead from galena by self-reduction process.
View SolutionWrite the chemical equations involved in the extraction of lead from galena by self-reduction process.
View SolutionWrite the chemical equations involved in the leaching of bauxite ore to prepare pure alumina.
View SolutionWrite chemical equation involved in Cannizzaro's reaction.
View Solution
