Given, H2(2)+Br2(g)→2HBr(g) , Δh∘1 and standerd enthalpy of condensation of bromine is ΔH∘2 , standard enthalpy of formation of HBr at 25∘C is
ΔH∘1/2
ΔH∘1/2+ΔH∘2
ΔH∘1−ΔH∘2/2 s
(ΔH∘1−ΔH∘2)/2
The correct Answer is:D
H2(g)+Br2(g)→2HBr(g),ΔH=ΔH∘1
Br2(g)→Br2(l),ΔH=ΔH∘2
Subtracting equation (ii) from equation (i), we get
H2(g)+Br(l)→2HBr(g),ΔH=Δ∘1−ΔH∘2
Required equation, 12H2(g)+12Br2(l)→HBr(g) , Δ=[ΔH∘1=ΔH∘22]
Similar Questions
Knowledge Check
Given, H2(g)+Br2(g)→2HBr(g),ΔH01 and standard enthalpy of condensaion of bromine is ΔH02, standard enthalpy of formation of HBr at 25∘C is
AΔH01/2BΔH01/2+ΔH02CΔH01/2−ΔH02D(ΔH01−ΔH02)/2Given,
H2(g)+Br2(g)→2HBr(g),ΔH∘1
and standard enthalpy of condensation of bromine is ΔH∘2, standard enthalpy of formation of HBr at 25∘ C is :AΔH∘12BΔH∘12+ΔH∘2CΔH∘12−ΔH∘2D(ΔH∘1−ΔH∘2)2Given H2(g)+Br2(g)→2HBr(g),ΔH∘1 and standard enthalpy of condensation of bromine is ΔH, standard enthalpy of formation of HBr at 25∘C is:
AΔH∘12BΔH∘12+ΔH∘2CΔH∘12−ΔH∘2DΔH∘1−ΔH∘22
Given N2(g)+3H(g)=2NH3(g),ΔH∘=22 kcal. The standard enthalpy of formation of NH3 gas is
View SolutionThe enthalpy change for the following process are listed below:
(a) Cl2(g)→2Cl(g),ΔH=242.3kJmol−1
(b) I2(g)→2I(g),ΔH=151.0kJmol−1
(c) ICl(g)→I(g)+Cl(g),ΔH=211.3kJmol−1
(d) I2(s)→I2(g),ΔH=62.76kJmol−1
If standard state of iodine and chloride are I2(s) and Cl2(g) , the standard enthalpy of formation for ICl(g) is :
View SolutionH2(g)+12O2(g)→H2O(l),
ΔH at 298K=−285.8kJ
The molar enthalpy of vaporization of water at 1 atm and 25∘C is 44 kJ. The standard enthalpy of formation of 1 mole of water vapour at 25∘C is
View SolutionLiquid bromine boils at 332.7.K Estimate the enthalpy of formation of Br2(g)∈Kj.Mol−1
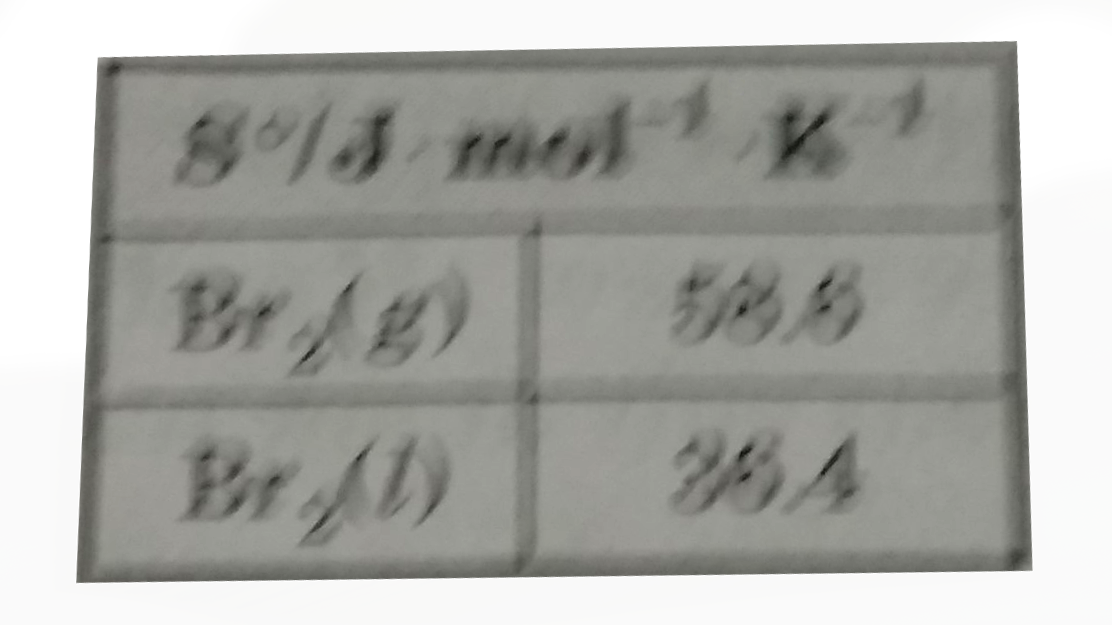
View SolutionFor a reaction, C(s)+O2(g)→CO2(g)
What is the relation between enthalpy of reaction (ΔHr), enthalpy of formation of CO2(ΔHf) and enthalpy of combustion of carbon (ΔHcomb) ?
View Solution
