In the following reaction
2I−+Cr2O2−7+14H+→I2+2Cl3+7H2O
Unbalanced parts are
H+, H2O
Cr2O2−7, Cr3+
I−, I2
None of them are balanced
The correct Answer is:C
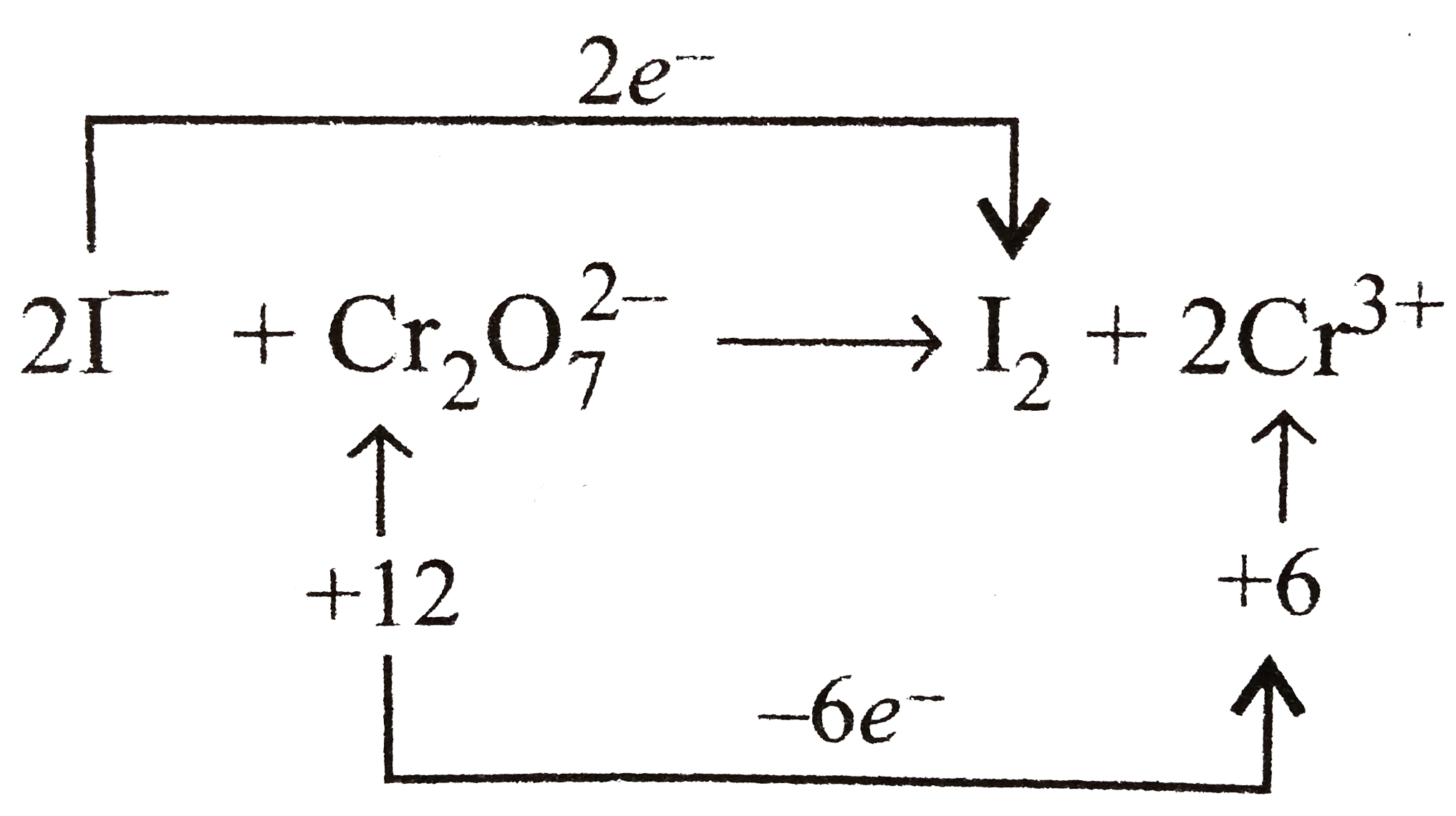
(I) Cr2O2−7+6e−→2Cr3+
(II) 2I−→I2+2e−
To balance electron (II) is to be multiplied by (3). Thus,
6I–––−+Cr2O2−7+14H+→3I–––2+2Cr3++7H2O
Thus, I− and I2 are not balanced.
Similar Questions
Complete the following reactions
(i) O2−2+H2O→ (ii) O−2+H2O→
View SolutionBalance the following equations by ion electron (half reaction) method for each of the following equations:
a. Cr2O2−7+Fe2+→cr3++Fe3++H2O
b. H2O2+IӨ+H⊕→H2O+2
c. AsO3−3+H⊕+IOӨ3→AsO3−4+IӨ (in acid medium)
d. Cr2O2−7+H⊕+ClӨ→3Cr3++Cl2+H2O
e. MnOӨ4+Fe2+→Mn2++Fe3+ (in alkaline medium)
View Solution
Knowledge Check
In the reaction:
Cr2O2−7+14H⊕+6IӨ→2Cr3++3H2O+3I2
Which element is reduced?
ACrBHCODIThe values of n in the following equation is
Cr2O2−7+14H++nFe2+→2Cr3−+nFe3++7H2OA4B3C7D6For reaction :
Cr2O−27+14H+→2Cr+3+7H2O, How many e−s are requiredA3B4C5D6
The value of n in the following equation is
Cr2O2−7+14H++ne→2Cr3++7H2O+
View SolutionThe equivalent mass of HCl in the following reaction :
K2Cr2O7+14HCl→2KCl+2CrCl3+3Cl2+7H2O would be
View SolutionIn the reaction:
Cr2O2−7+14H⊕+6IӨ→2Cr3++3H2O+3I2
Which element is reduced?
View SolutionWhich substance is serving as a reducing agent in the following reaction?
14H++Cr2O2−7+3Ni→2Cr3++7H2O+3Ni2+
View SolutionThe value of n in the equation,
Cr2O2−7+14H++≠−→2Cr3++7H2O is :
View Solution
