In diborane
A
2 bridge hydrogen atoms and four terminal hydrogen atoms are present
B
3 bridge and three terminal hydrogen atoms present
C
4 bridge hydrogen atoms and two terminal hydrogen atoms are present
D
1 baridge hydrogen atoms and 1 terminal hydrogen are present
Video Solution
Text Solution
The correct Answer is:A
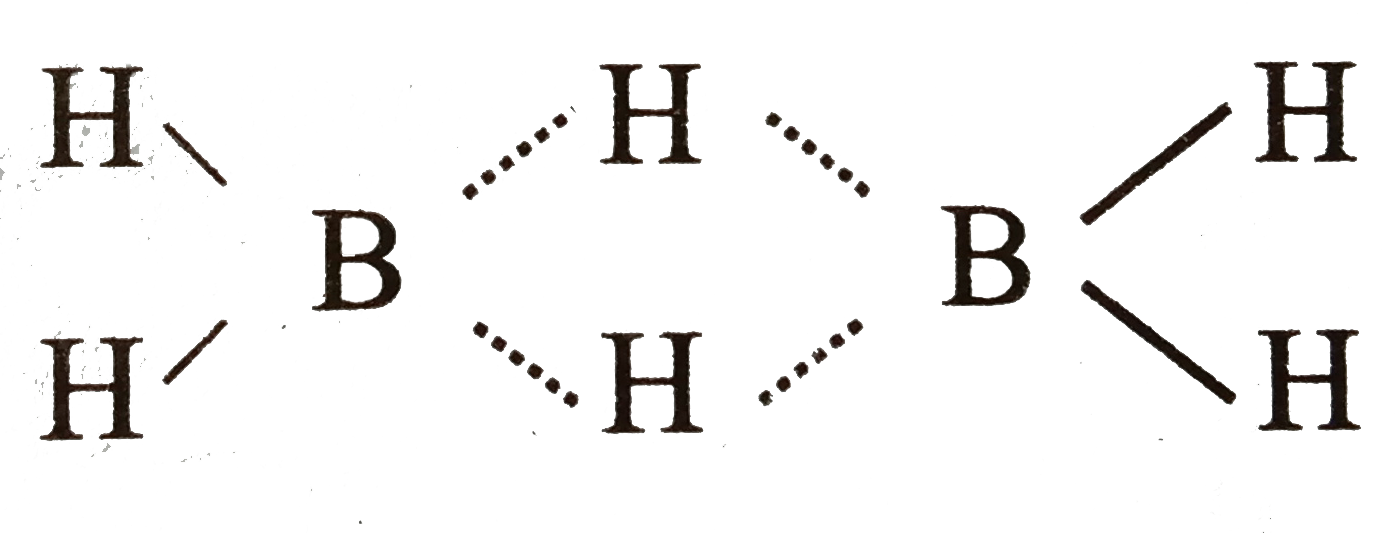
Two bridge hydrogen atoms and four terminal hydrogen atoms are present.
|
Similar Questions
Knowledge Check
The prdouct/s formed when diborane is hydrolysed is/are
AB2O3 and H3BO3BB2O3 onlyCH3BO3 and H2DH3BO3 onlyThe prdouct/s formed when diborane is hydrolysed is/are
AB2O3 and H3BO3BB2O3 onlyCH3BO3 and H2DH3BO3 onlyWhat happen when diborane reacts with Lewis bases?
AIt forms boron trihydride (BH3) due to cleavage.BIt undergoes cleavage to give borane adduct BH3L (Where, L = Lewis base)CIt oxidises to give B2O3DIt does not react with Lewis bases
The number of atoms involved in bridged bonds in one diborane molecule is
View SolutionWhich one of the following statements is not true regarding diborane?
View SolutionHydroboration reactions are usually carried out in ethers. The reagent diborane is commercially available in
View SolutionIn the structure of diborane
View SolutionIn the structure of diborane
View Solution
