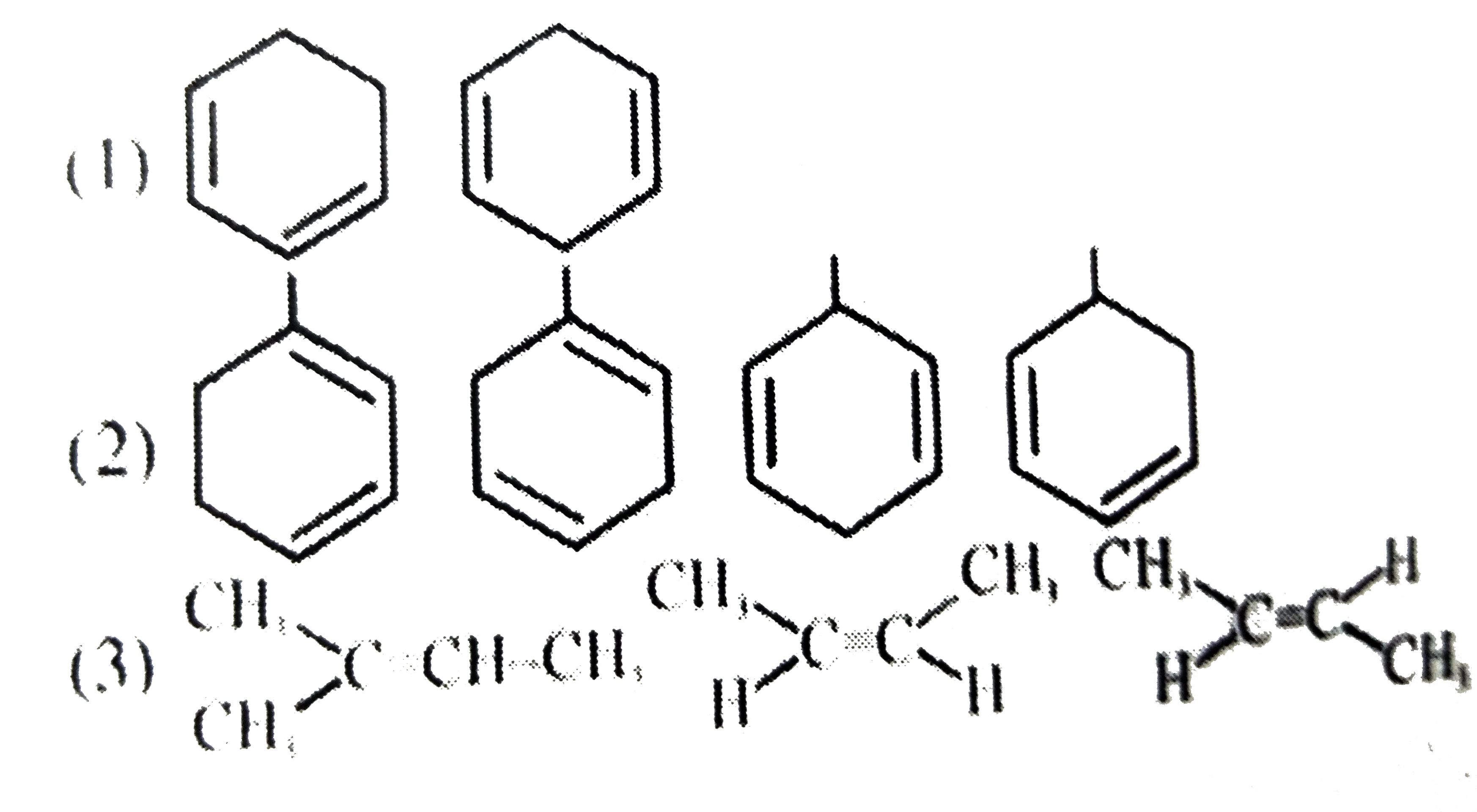
The minimum magnitude of heat of hydrogenation per mole of molecule is of:
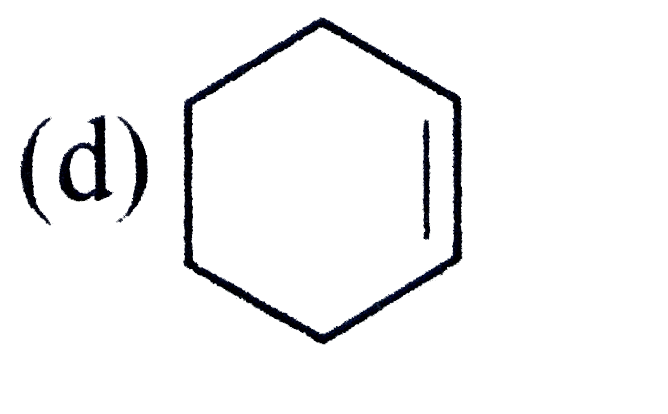
The correct Answer is:D
Step by step video, text & image solution for The minimum magnitude of heat of hydrogenation per mole of molecule is of: by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
The structure of hydrogen peroxide molecule is
AlinearBhalf open book likeCclosed book likeDcyclic.
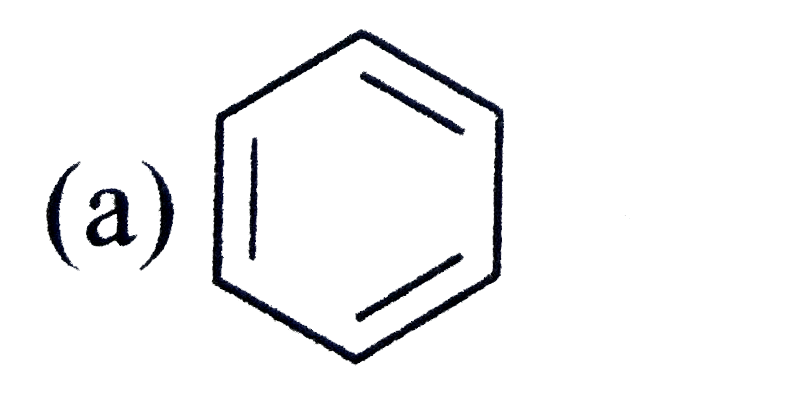
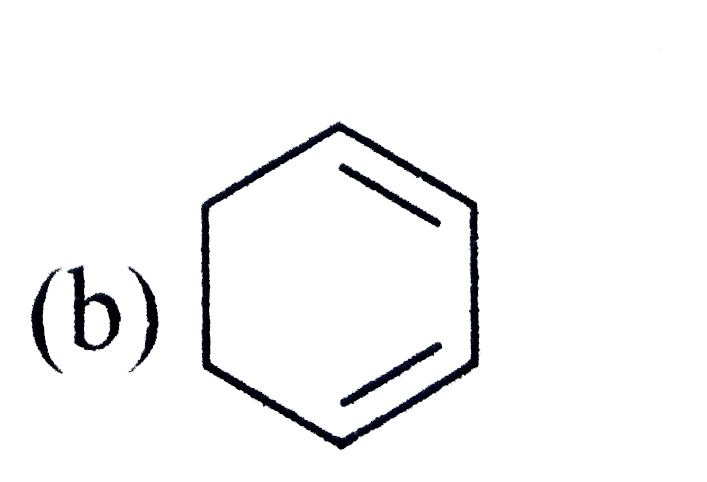
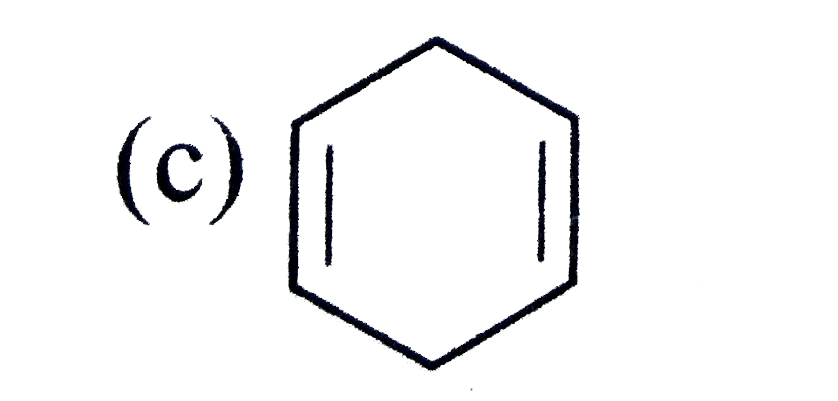
The correct order of heat of hydrogenation of given molecules is:Ad>c>a>bBd>c>b>aCb>a>c>dDd>a>c>bIncorrect order of Heat of hydrogenation per π-bond is
A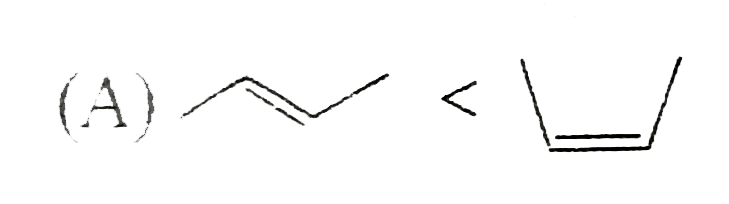 B
B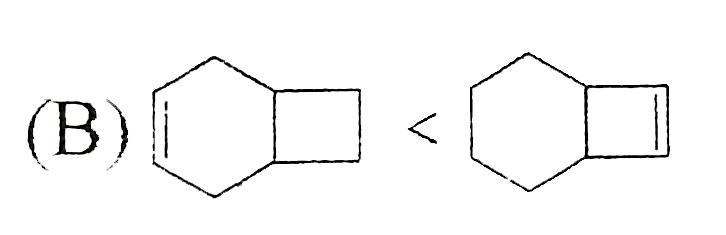 C
C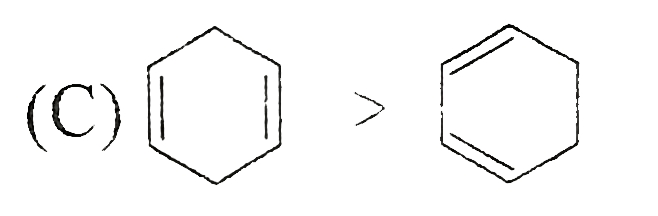 D
D
In three moles of ethane(C2H6), calculate the following:
(i) Number of moles of carbon atoms.
(ii) Number of moles of hydrogen atoms.
(iii) Number of molecules of ethane.
View SolutionIn three moles of ethane(C2H6), calculate the following:
(i) Number of moles of carbon atoms.
(ii) Number of moles of hydrogen atoms.
(iii) Number of molecules of ethane.
View SolutionWhich one of the following has the smalJes, heat of hydrogenation per mole of the compound ?
View SolutionThe number of electrons in a mole of hydrogen molecule is
View SolutionThe number of electrons in a mole of hydrogen molecule is
View Solution