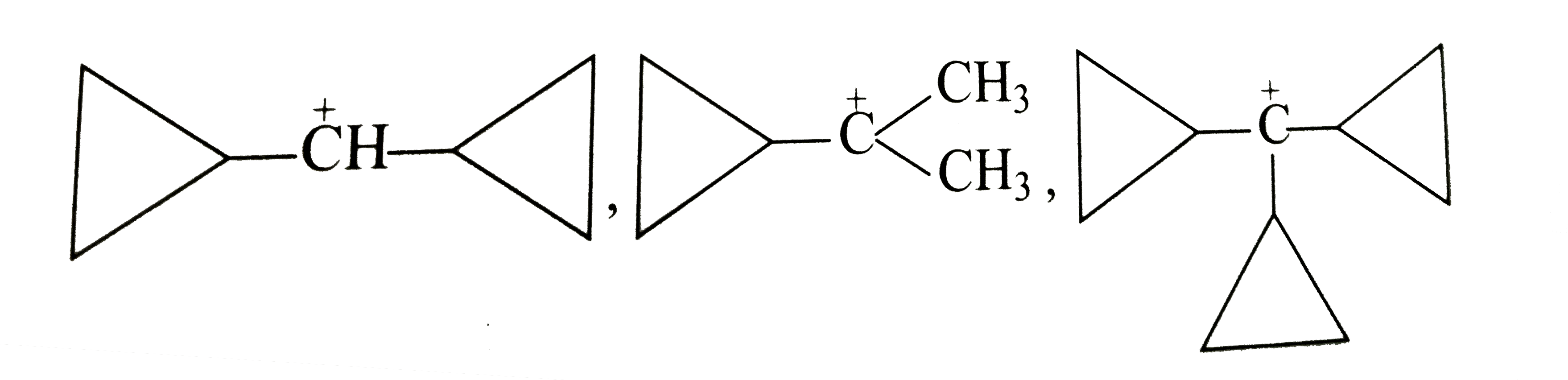
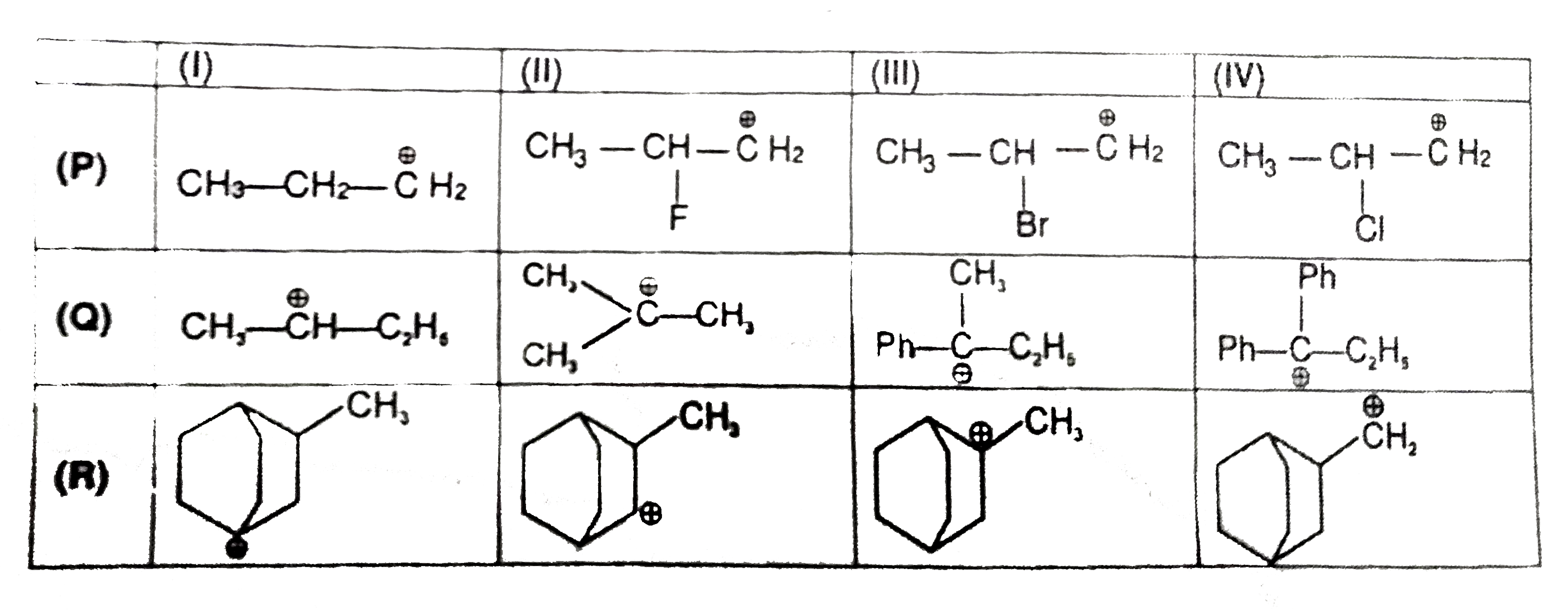
Arrange the following carbocation in decreasing order of stability.

A
I>III>II>IV
B
II>I>III>IV
C
IV>III>II>I
D
III>IV>II>I
Video Solution
Text Solution
The correct Answer is:B
II is stabilised by resonance effect, most stable followed by I which is stabilised only by inductive and hyperconjugation effect. IV is least stable due to greater electron withdrawing power of −NO2 than −CN.
|
Similar Questions
Knowledge Check
Arrange the following carbanions in decreasing order of stability:
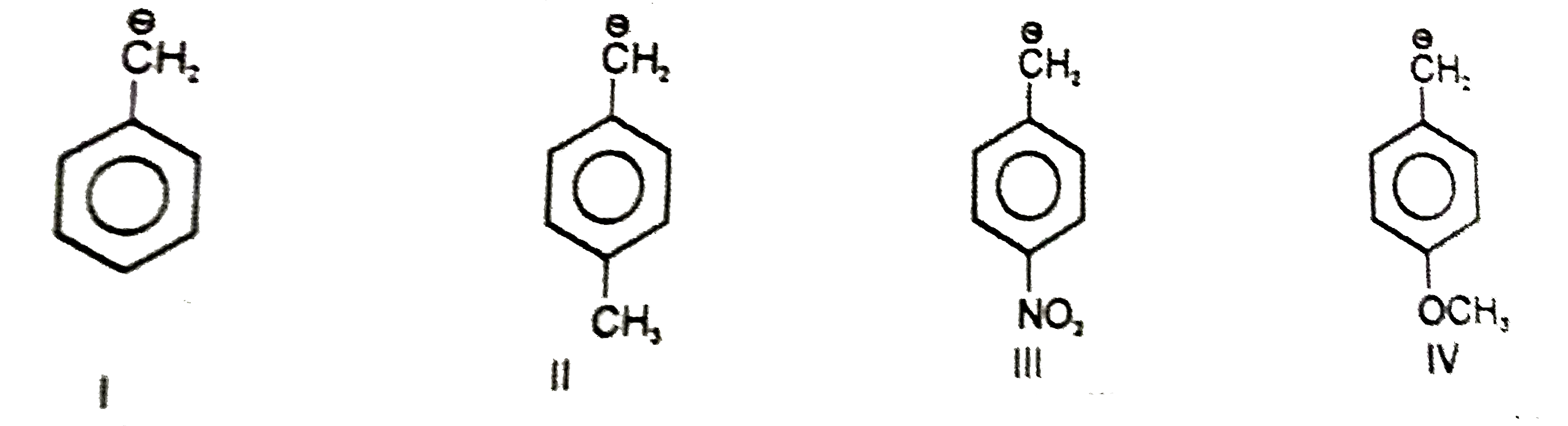 AIII>I>IV>IIBIII>II>I>IVCI>III>II>IVDIII>I>II>IV
AIII>I>IV>IIBIII>II>I>IVCI>III>II>IVDIII>I>II>IVArrange the following carbanions in increasing order of stability:
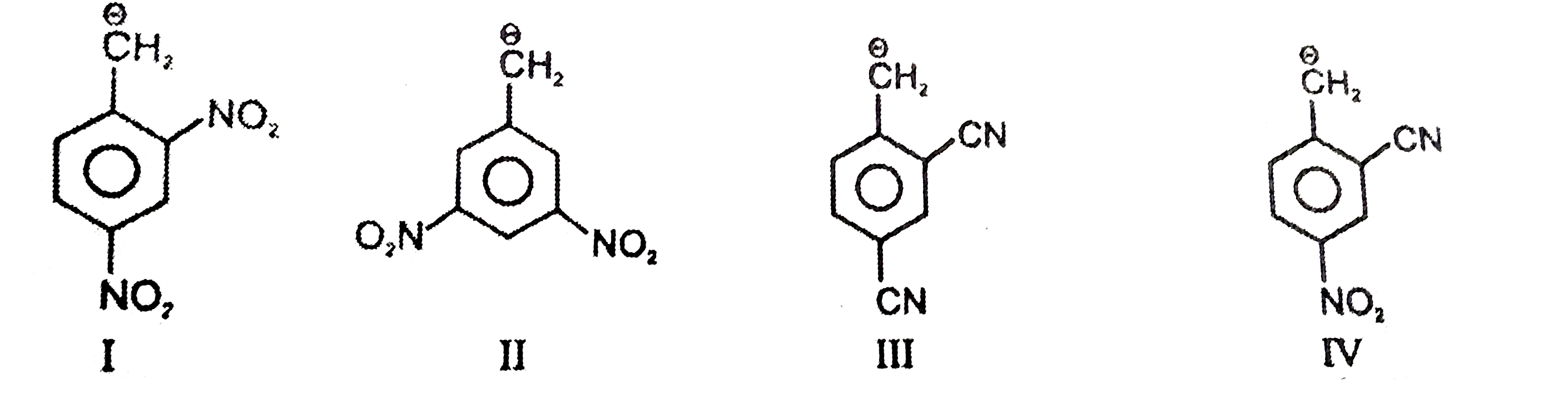 AIII>VI>I>IIBI>III>IV>IICI>II>III>IVDI>IV>III>II
AIII>VI>I>IIBI>III>IV>IICI>II>III>IVDI>IV>III>IIArrange following carbocation in the decreasing order of stability
(i) CH3−+CH−CH3
(ii) CH3−+CH−O−CH3
(iii) CH3−+CH−CO−CH3A(iii)>(ii)>(i)B(i)>(ii)>(iii)C(i)>(ii)>(iii)D(i)<(ii)<(iii)
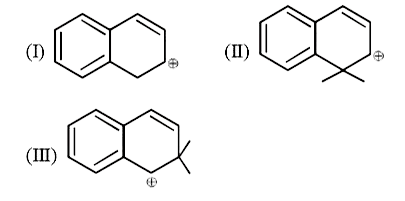
Arrange the given carbocation in decreasing order of stability :
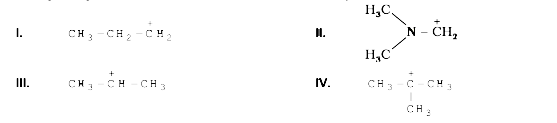
The correct choice is :
View SolutionArrange the following free radicals in decreasing order of stability :
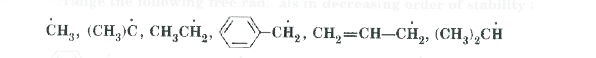
View SolutionArrange the following free radicals in decreasing order of stability:
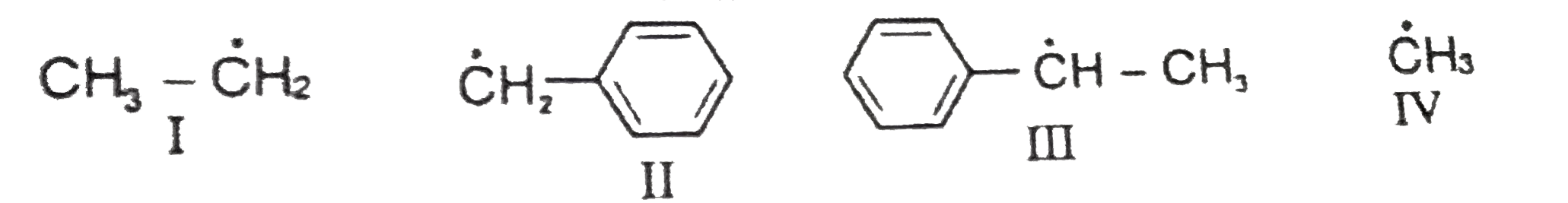
View SolutionArrange the following gem diols in decreasing order of stability :
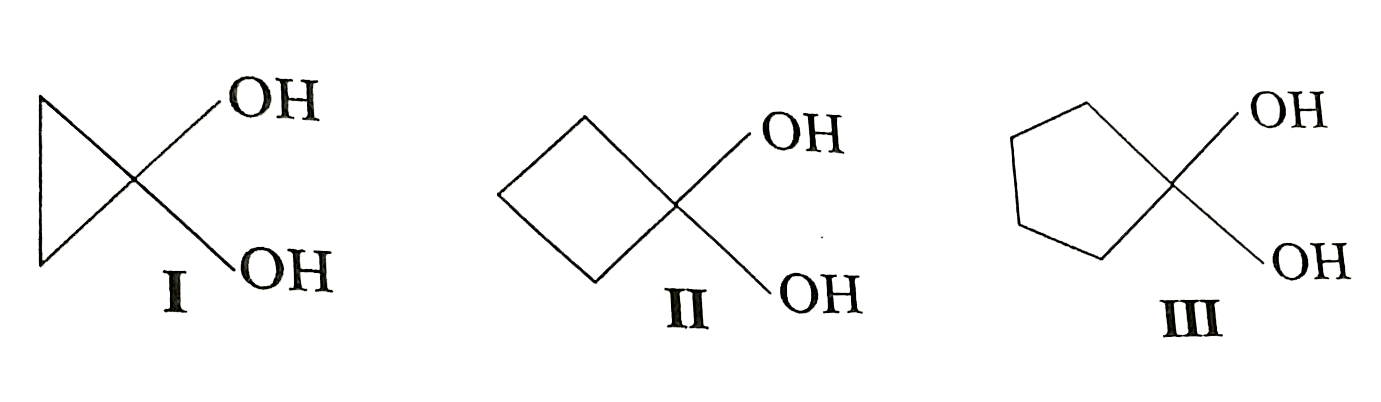
View SolutionArrange the following carbocations in the increasing order of their stability.
View Solution