Which of the following shows functional isomerism?
CH3CH2Cl and CH3CH2Br
CH3CH2Br and CH2BrCH2Br
C2H5OC2H5 and CH3OC3H7
CH3CH2CHO and CH3COCH3
The correct Answer is:D
To determine which of the given compounds shows functional isomerism, we need to analyze each option based on the definition of functional isomerism. Functional isomerism occurs when compounds have the same molecular formula but different functional groups.
1. Identify the Compounds:
We have four parts (A, B, C, D) to analyze. Each part represents a different compound or set of compounds.
2. Analyze Part A:
- Compounds: CH3CH2Cl and CH3CH2Br
- Both compounds have the same molecular formula (C2H5X where X is Cl or Br).
- However, they differ only in the halogen atom (Cl vs. Br), which does not change the functional group.
- Conclusion: Does not show functional isomerism.
3. Analyze Part B:
- Compounds: CH3CH2Br and CH2BrCH2Br
- The first compound has one Br, while the second has two Br atoms.
- They have different molecular formulas (C2H5Br vs. C2H4Br2).
- Conclusion: Does not show isomerism as they are different compounds.
4. Analyze Part C:
- Compounds: C2H5O-C2H5 (ether) and CH3O-C3H7 (another ether).
- Both compounds are ethers and have the same functional group (ether).
- Conclusion: Does not show functional isomerism.
5. Analyze Part D:
- Compounds: CH3CH2CHO (aldehyde) and CH3COCH3 (ketone).
- Both compounds have the same molecular formula (C3H6O) but different functional groups (aldehyde vs. ketone).
- Conclusion: Shows functional isomerism.
6. Final Answer:
- The correct option that shows functional isomerism is Part D (CH3CH2CHO and CH3COCH3).
We have four parts (A, B, C, D) to analyze. Each part represents a different compound or set of compounds.
- Compounds: CH3CH2Cl and CH3CH2Br
- Both compounds have the same molecular formula (C2H5X where X is Cl or Br).
- However, they differ only in the halogen atom (Cl vs. Br), which does not change the functional group.
- Conclusion: Does not show functional isomerism.
- Compounds: CH3CH2Br and CH2BrCH2Br
- The first compound has one Br, while the second has two Br atoms.
- They have different molecular formulas (C2H5Br vs. C2H4Br2).
- Conclusion: Does not show isomerism as they are different compounds.
- Compounds: C2H5O-C2H5 (ether) and CH3O-C3H7 (another ether).
- Both compounds are ethers and have the same functional group (ether).
- Conclusion: Does not show functional isomerism.
- Compounds: CH3CH2CHO (aldehyde) and CH3COCH3 (ketone).
- Both compounds have the same molecular formula (C3H6O) but different functional groups (aldehyde vs. ketone).
- Conclusion: Shows functional isomerism.
- The correct option that shows functional isomerism is Part D (CH3CH2CHO and CH3COCH3).
Similar Questions
Knowledge Check
Which of the following shows optical isomerism?
AButan-1-olBButan-2-olCButeneDBut-2-enolWhich of the following shows geometrical isomerism?
A1,2-dichloroetheneB1,2-dimethylcyclopropaneC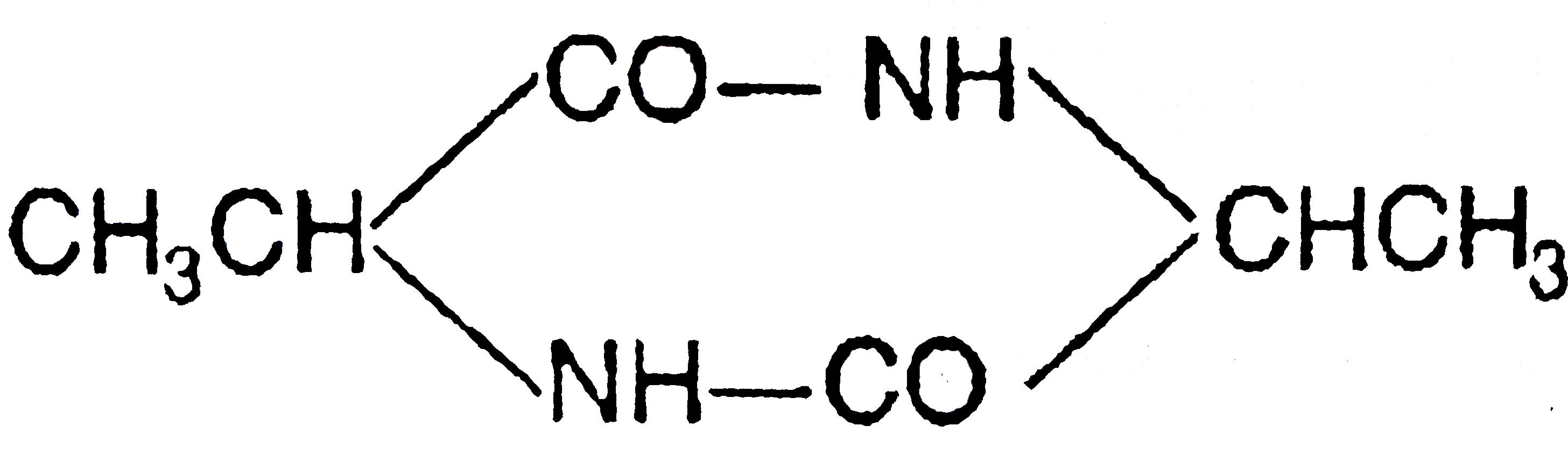 DAll of the above
DAll of the aboveWhich of the following show geometrical isomerism
A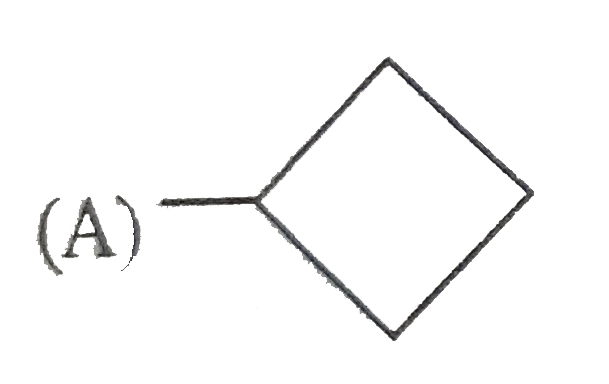 B
B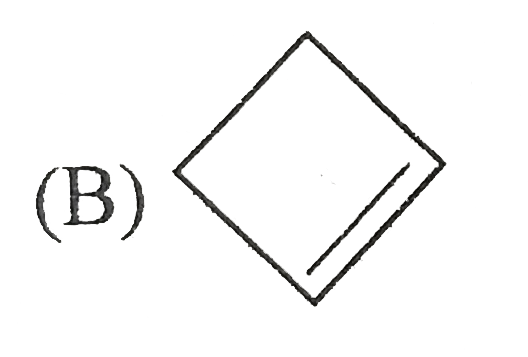 C
C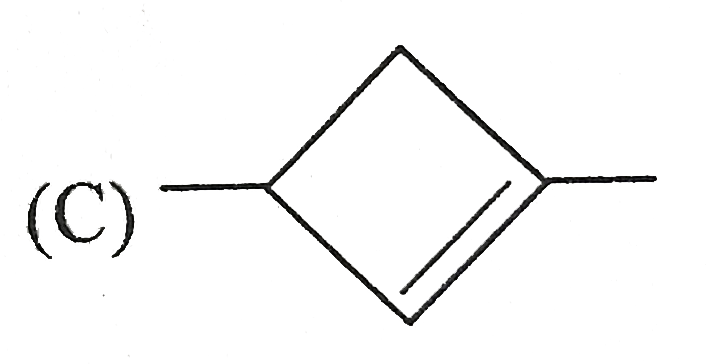 D
D
Which of the following show geometrical isomerism?
View SolutionWhich of the following represents functional isomerism ?
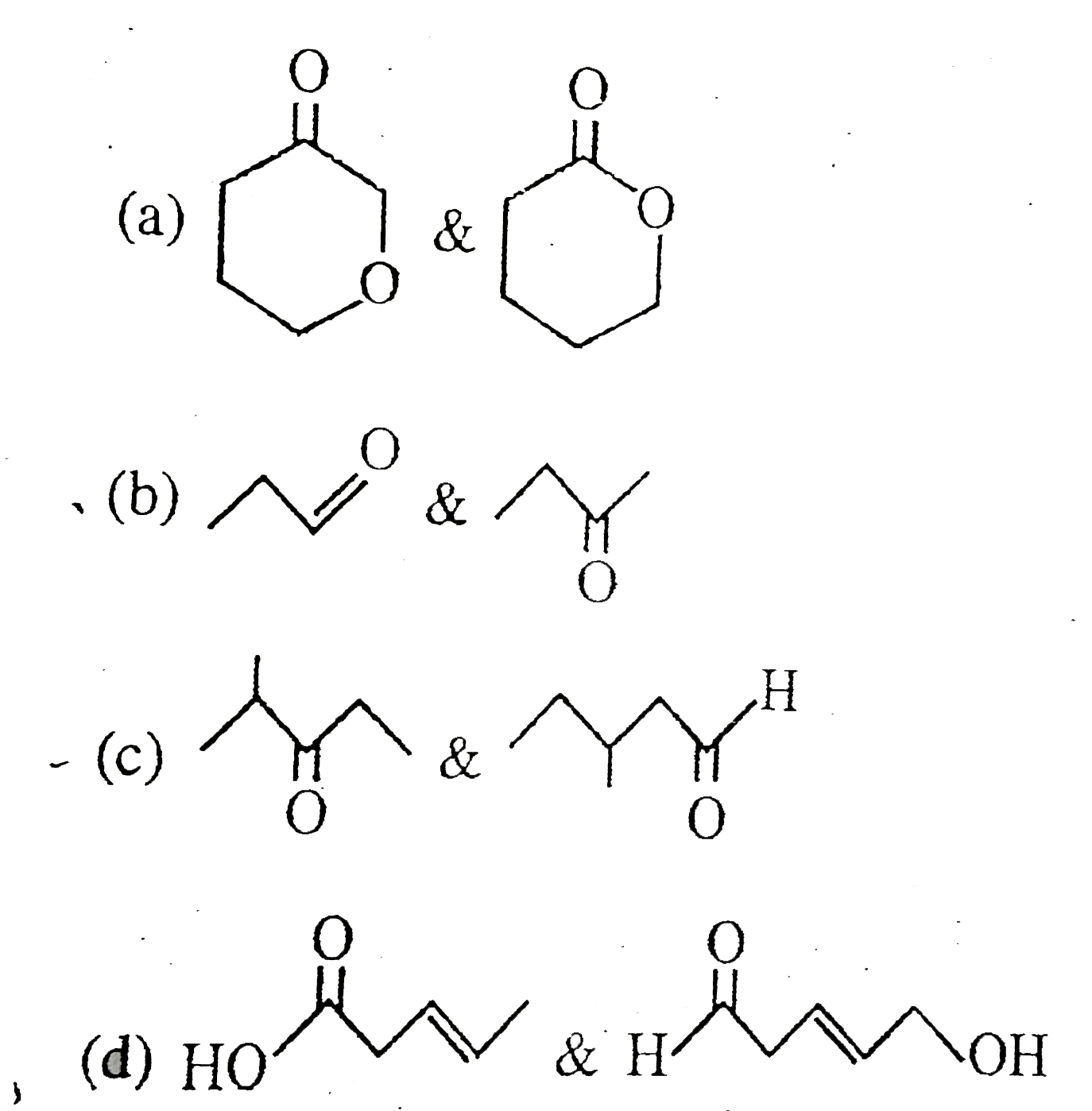
View SolutionWhich of the following shows geometrical isomerism.
View SolutionWhich of the following show geometerical isomerism
View SolutionWhich of the following will show optical isomerism? .
View Solution

