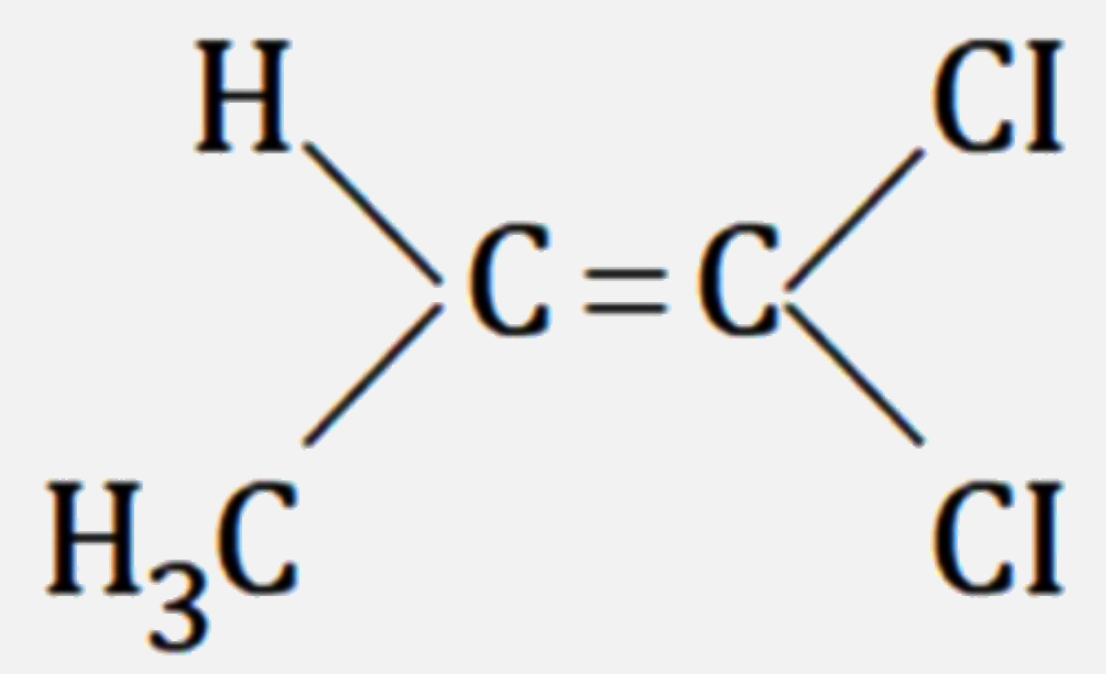Geometrical isomerism results because molecule has
A centre of symmetry
A plane of symmetry
The capacity to rotate the plane of polarized light
Two dissimilar groups attached to a double bond carbon atom
The correct Answer is:D
To determine why geometrical isomerism occurs in certain molecules, we can follow these steps:
1. Understand Geometrical Isomerism:
Geometrical isomerism is a type of stereoisomerism that arises due to the different spatial arrangements of groups around a double bond or a ring structure.
2. Identify the Requirement for Geometrical Isomerism:
For geometrical isomerism to occur, there must be a carbon-carbon double bond (C=C) or a cyclic structure. This is because the double bond or the ring restricts rotation, leading to distinct configurations.
3. Check for Dissimilar Groups:
At least one of the carbon atoms involved in the double bond must be bonded to two different groups (dissimilar groups). For example, if we have a double bond between two carbon atoms, one carbon might be attached to a methyl group (CH₃) and a chlorine atom (Cl), while the other carbon is attached to a hydrogen atom (H) and another methyl group (CH₃).
4. Identify the Isomers:
The two main types of geometrical isomers are:
- Cis Isomer: The two similar groups are on the same side of the double bond.
- Trans Isomer: The two similar groups are on opposite sides of the double bond.
5. Conclusion:
Therefore, geometrical isomerism results because the molecule has at least one carbon-carbon double bond with two dissimilar groups attached to the carbon atoms involved in the double bond.
Final Answer:
Geometrical isomerism results because the molecule has a carbon-carbon double bond with two dissimilar groups attached to it.
---
Geometrical isomerism is a type of stereoisomerism that arises due to the different spatial arrangements of groups around a double bond or a ring structure.
For geometrical isomerism to occur, there must be a carbon-carbon double bond (C=C) or a cyclic structure. This is because the double bond or the ring restricts rotation, leading to distinct configurations.
At least one of the carbon atoms involved in the double bond must be bonded to two different groups (dissimilar groups). For example, if we have a double bond between two carbon atoms, one carbon might be attached to a methyl group (CH₃) and a chlorine atom (Cl), while the other carbon is attached to a hydrogen atom (H) and another methyl group (CH₃).
The two main types of geometrical isomers are:
- Cis Isomer: The two similar groups are on the same side of the double bond.
- Trans Isomer: The two similar groups are on opposite sides of the double bond.
Therefore, geometrical isomerism results because the molecule has at least one carbon-carbon double bond with two dissimilar groups attached to the carbon atoms involved in the double bond.
Geometrical isomerism results because the molecule has a carbon-carbon double bond with two dissimilar groups attached to it.
Similar Questions
Knowledge Check
Geometrical isomerism is caused
Aby restricted rotation around C=C bondBby the presence of one asymmetric carbon atomCdue to the differennt groups attached to the same functional groupDby swing of hydrogenn atom between two divalent atoms.Geometrical isomerism is show by
A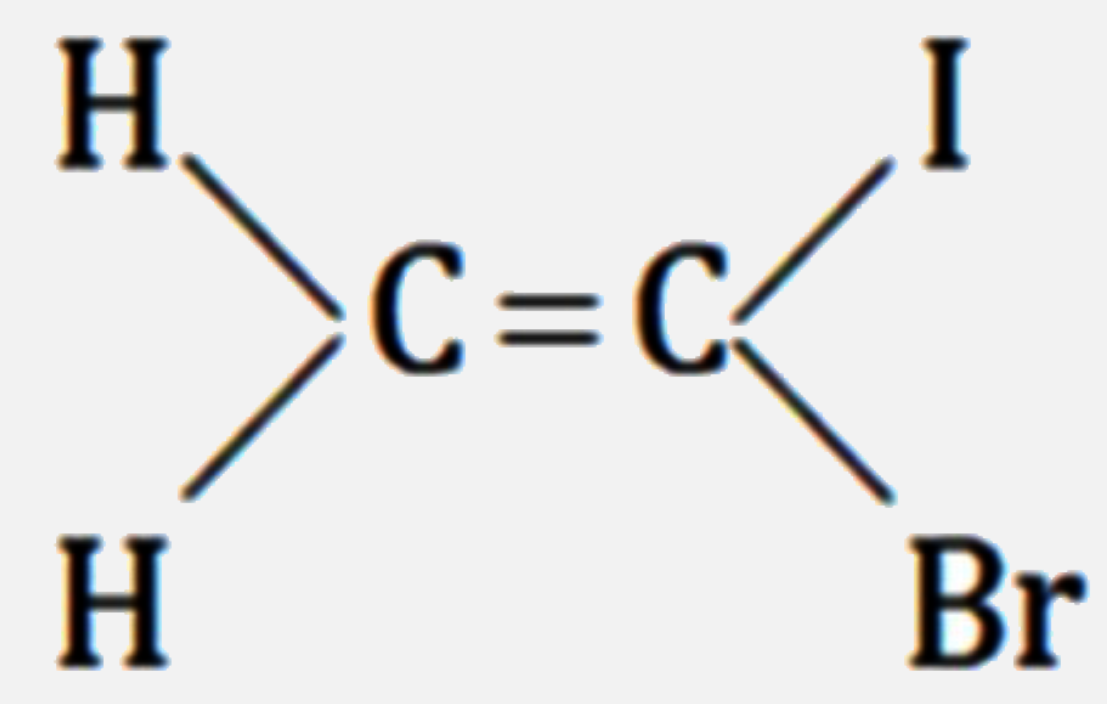 B
B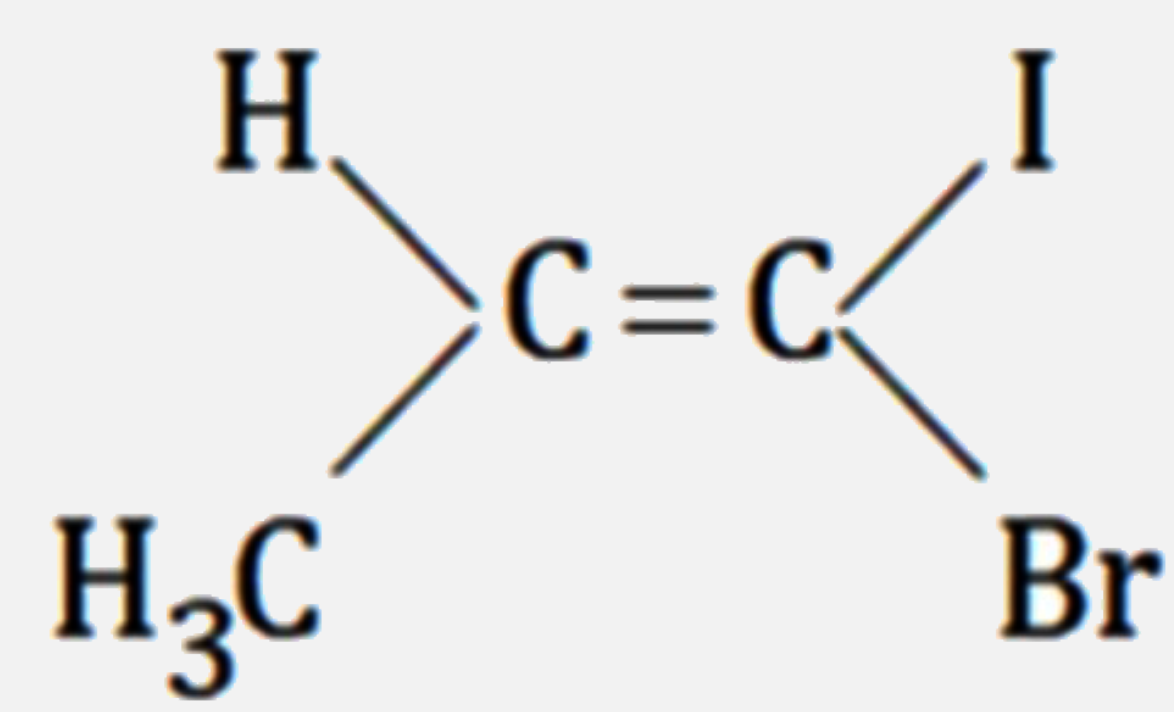 C
C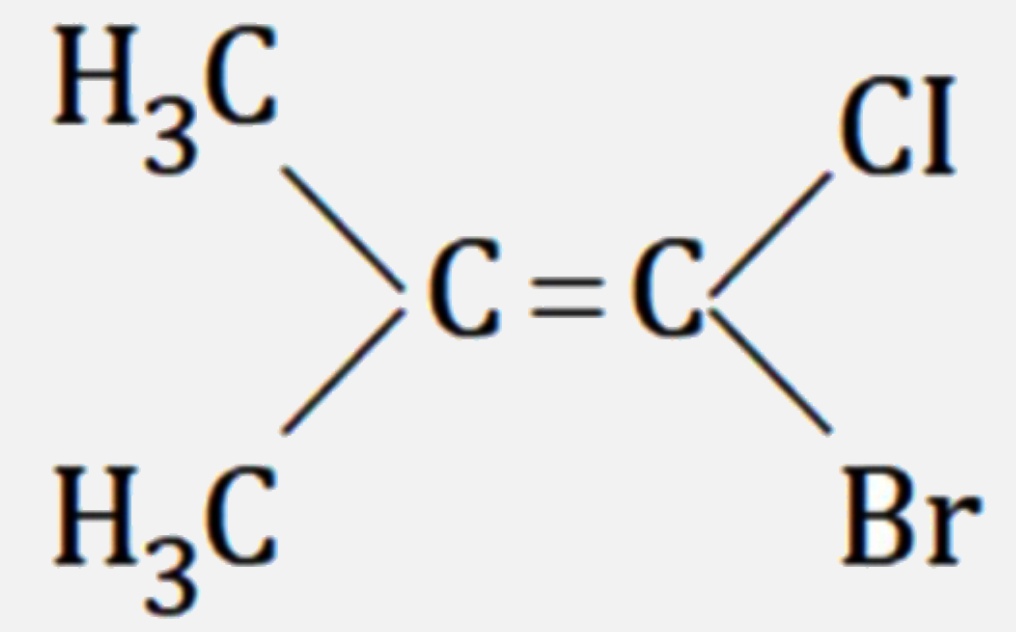 D
D