Number of chiral carbon present in the following compound:
CH3−C∣OHH−CH2−C∣BrH−C∣C2H5H−CH3
2
3
4
5
The correct Answer is:B
To determine the number of chiral carbons in the given compound, we will analyze each carbon atom in the structure and check if it meets the criteria for chirality. A carbon atom is considered chiral if it is attached to four different groups.
1. Identify the Carbon Atoms:
The structure of the compound is:
CH3−OH|CH|CH2−Br|CH−C2H5|CH−CH3
We will examine each carbon atom in the compound.
2. Examine the First Carbon (CH3):
- This carbon is attached to three hydrogen atoms (3H).
- Since all three substituents are the same (H), this carbon is not chiral.
3. Examine the Second Carbon (attached to OH):
- This carbon is attached to:
- OH (hydroxyl group)
- CH3 (methyl group)
- H (hydrogen atom)
- CH (next carbon in the chain)
- All four substituents are different, making this carbon chiral.
4. Examine the Third Carbon (CH):
- This carbon is attached to:
- CH2 (next carbon in the chain)
- Br (bromine)
- H (hydrogen)
- CH (the carbon with OH)
- All four substituents are different, making this carbon chiral.
5. Examine the Fourth Carbon (C2H5):
- This carbon is attached to:
- CH2 (next carbon in the chain)
- CH3 (methyl group)
- H (hydrogen)
- CH (the carbon with OH)
- All four substituents are different, making this carbon chiral.
6. Examine the Fifth Carbon (CH):
- This carbon is attached to:
- CH3 (methyl group)
- CH2 (next carbon in the chain)
- H (hydrogen)
- CH3 (the carbon with 3H)
- Since it has two identical substituents (CH3), this carbon is not chiral.
Conclusion:
After analyzing all the carbon atoms in the compound, we find that there are three chiral carbons.
Final Answer:
The total number of chiral carbons in the compound is 3.
---
The structure of the compound is:
CH3−OH|CH|CH2−Br|CH−C2H5|CH−CH3
We will examine each carbon atom in the compound.
- This carbon is attached to three hydrogen atoms (3H).
- Since all three substituents are the same (H), this carbon is not chiral.
- This carbon is attached to:
- OH (hydroxyl group)
- CH3 (methyl group)
- H (hydrogen atom)
- CH (next carbon in the chain)
- All four substituents are different, making this carbon chiral.
- This carbon is attached to:
- CH2 (next carbon in the chain)
- Br (bromine)
- H (hydrogen)
- CH (the carbon with OH)
- All four substituents are different, making this carbon chiral.
- This carbon is attached to:
- CH2 (next carbon in the chain)
- CH3 (methyl group)
- H (hydrogen)
- CH (the carbon with OH)
- All four substituents are different, making this carbon chiral.
- This carbon is attached to:
- CH3 (methyl group)
- CH2 (next carbon in the chain)
- H (hydrogen)
- CH3 (the carbon with 3H)
- Since it has two identical substituents (CH3), this carbon is not chiral.
After analyzing all the carbon atoms in the compound, we find that there are three chiral carbons.
The total number of chiral carbons in the compound is 3.
Similar Questions
Knowledge Check
Identify number of chiral carbons in the following compounds
(I)H3C−CH∣OH−CH∣OH−CH2∣OH
(II)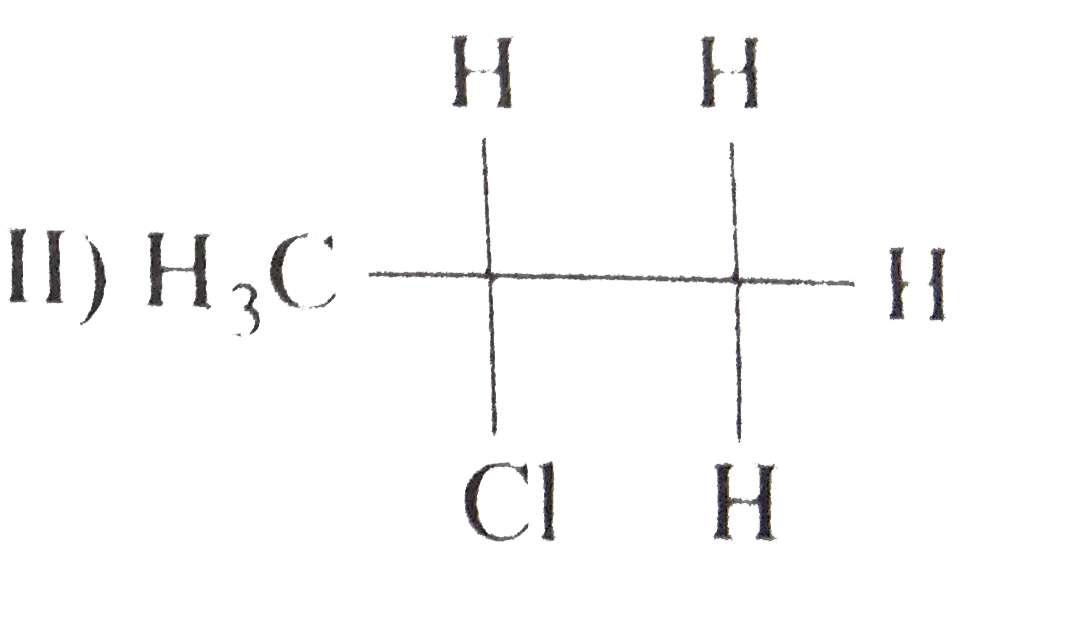
H3−CH∣CH3−CH∣CH3−CH2−CH2A1,2,1B1,1,2C2,0,1D2,1,1The IUPAC name of CH3−CH∣CI−CH∣Br−CH∣OH−CH3 is:
A3-Bromo-4-chloropentan-2-olB3-Bromo-2-chloro-4-hydroxypentaneC3-Bromo-2-chloropentane-4-olDnone of theseThe IUPAC name the compound
CH3−CH∣OH−CH∣OH−CH2∣OH isAButane -2, 3, 4-triolBButane-1, 2, 3-triolCPentane -1, 2, 3-triolD2, 3- dihydroxy butanlol
Give IUPAC names of the following compounds:
(a) CH3−C∣OHH−CH2−C∣OHH−C∣C2H5H−CH2CH3 (b) CH3−C∣OHH−C∣OHH−CH3
(c)CH3C∣OHHCH2C∣CH3HCH2CH2CH2CH3 (d) CH3C∣CH3H−C∣OHH−CH3∣C∣CH3−CH3
(e) CH3C∣OHHC∣OHHCH2OH (f) C6H5CH2OH
(g) CH3CH2CHCH4OH (h) CH3CH2CH2OH∣C∣C6H5CH2CH3
(i) CH3OH∣CHCH2OH∣CHC∣CH3H−CH3 (j)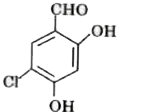
View SolutionWrite the IUPAC name of the following compound:
CH3−C∣BrH−CH2−C∣ClH−CH3
View SolutionGive the IUPAC name of the compound
CH3−C∣ClH−C∣BrH−C∣OHH−CH3
View SolutionWrite IUPAC name of CH3−C∣BrH−C∣CHOH−CH3
View SolutionThe IUPAC name for the compound
CH3−C∣OHH−CH2−C∣BrH−CHO, is
View Solution
