An organic compound has molecular formula C9H18. It is a saturated hydrocarbon and its all hydrogen atoms are identical. Its structural formula can be :
A
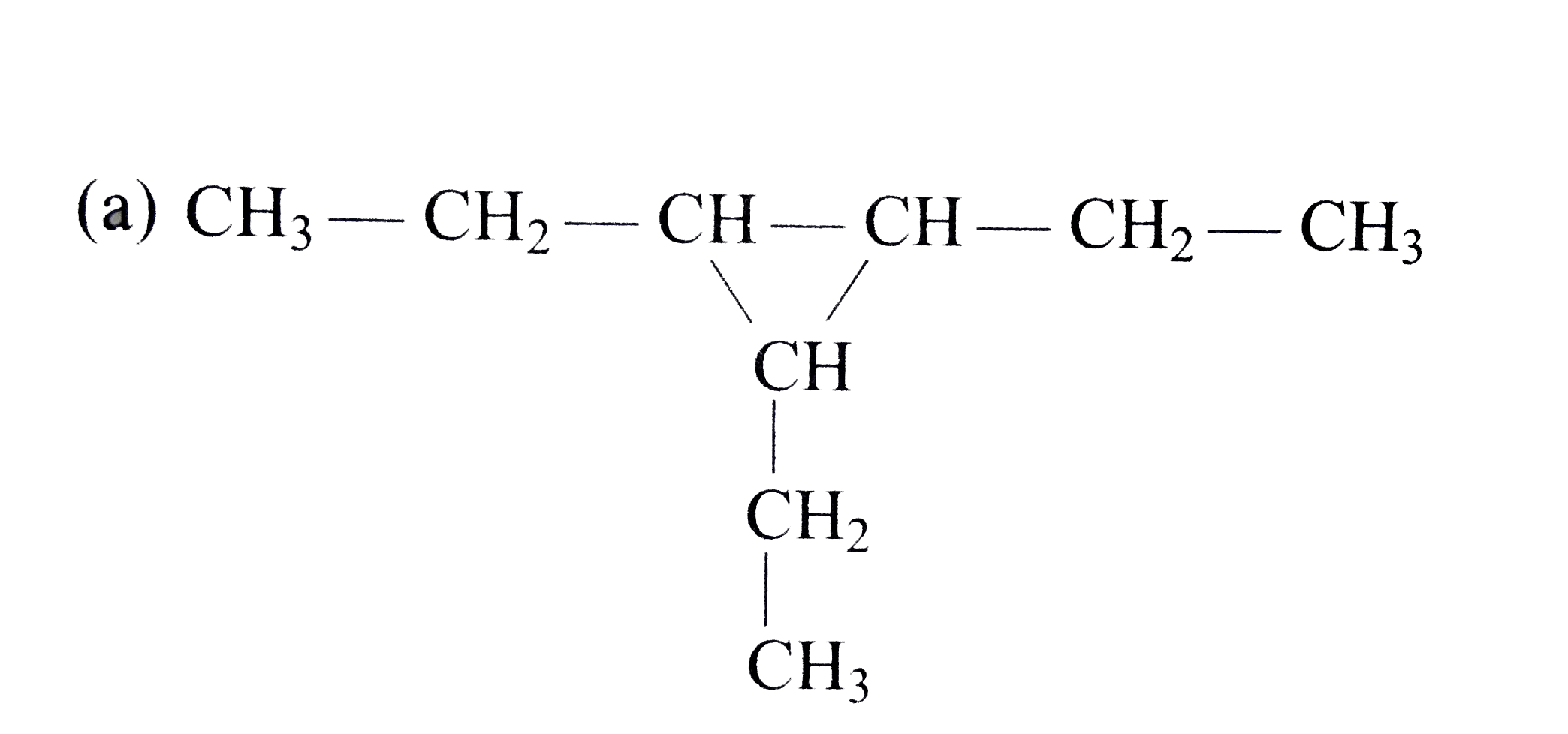
B
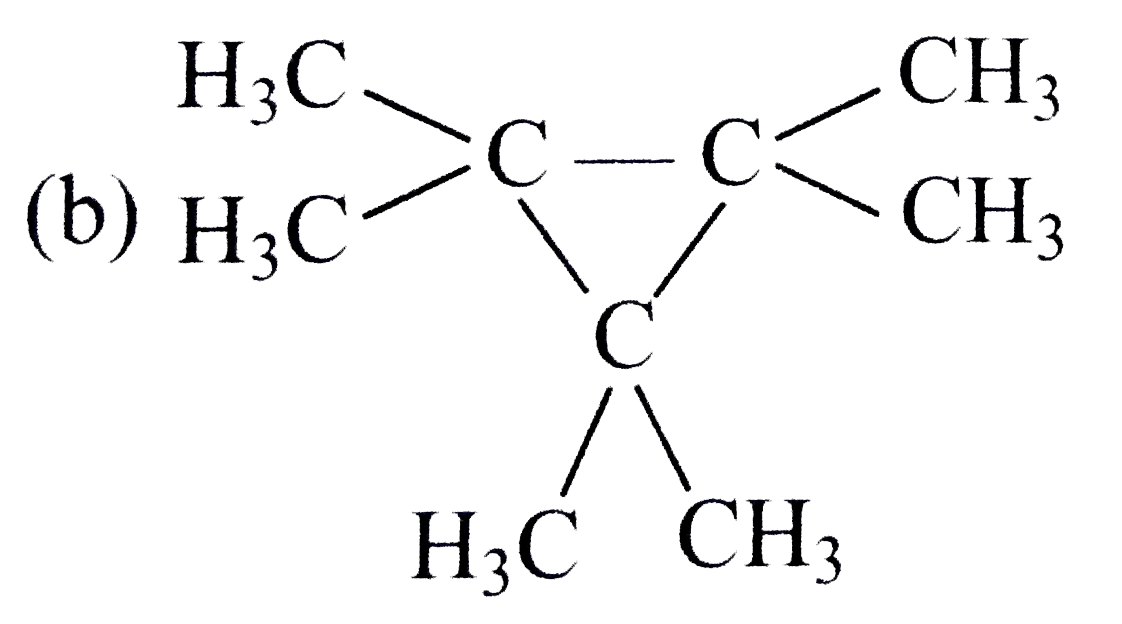
C
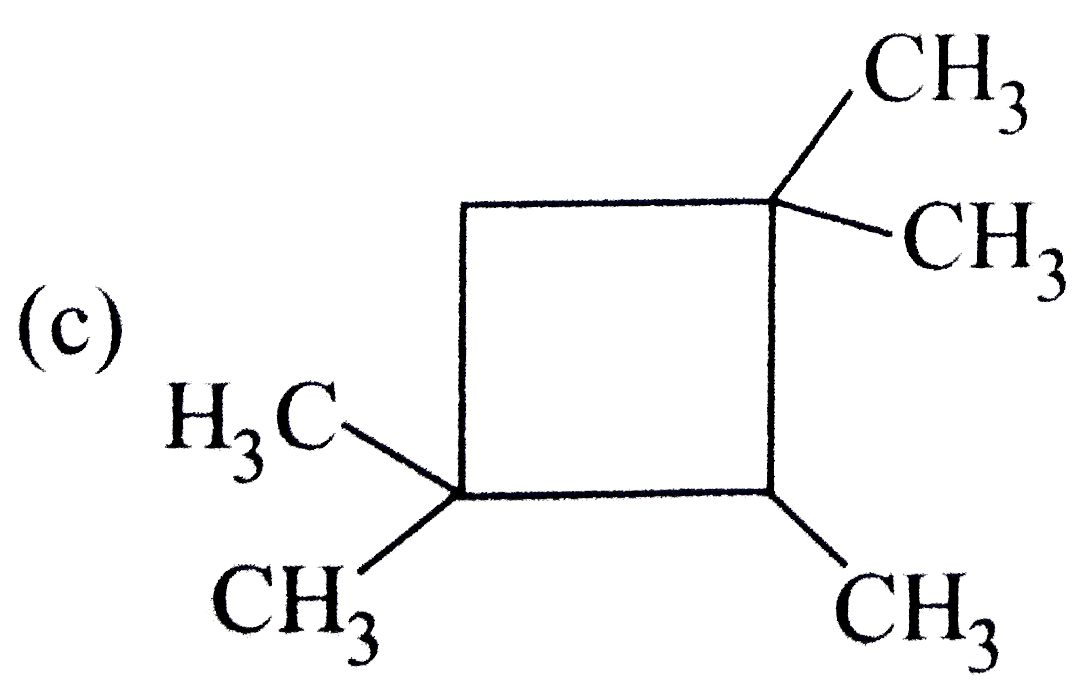
D
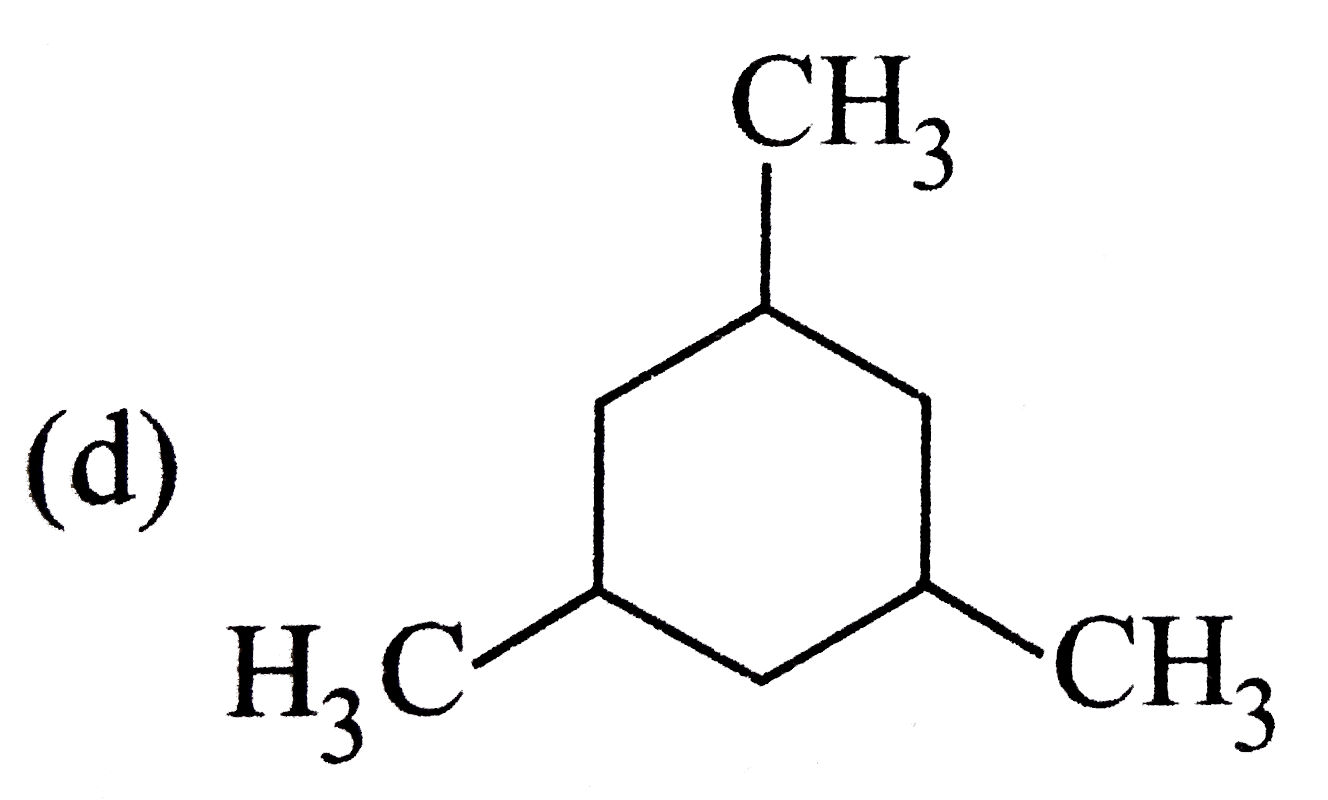
Video Solution
Text Solution
The correct Answer is:b
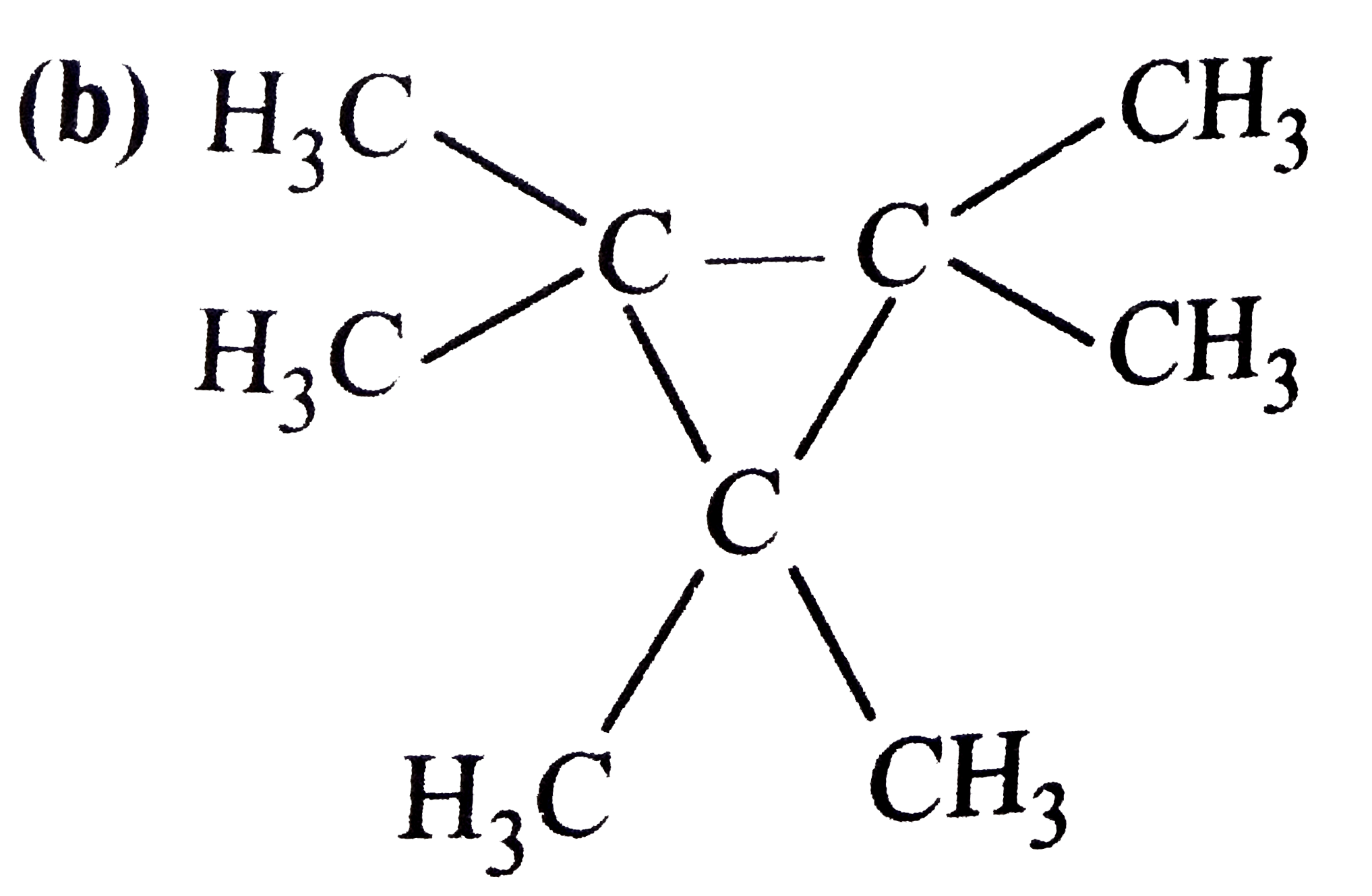
This structures has 1∘H− atoms and all are identical.

|
Similar Questions
Knowledge Check
A compound with molecular formula C6H14 has two tertiary carbons. Its IUPAC name is
An-hexaneB2-methyl pentaneC2,3-dimethyl butaneD2,3-dimethyl pentaneA compound with molecular formula C6H14 has two tertiary carbons. Its IUPAC name is
An-hexaneB2-methylpentaneC2, 3-dimethylbutaneD2, 3-dimethylpentaneThe structure of the compound with molecular formula C8H14 that has 12 secondary and 2 tertiary H atoms is:
A(a) Bicyclo [2.2.2] octaneB(b) 1,2-Dimethyl cyclohexaneC(c) Bicyclo [3.2.1] octaneD(d) Tricylo [2.2.2.0] octane
A hydrocarbon has three carbon atoms. Write down its molecular formulae as
alkane
View SolutionA hydrocarbon has three carbon atoms. Write down its molecular formulae as
alkene
View SolutionA hydrocarbon has three carbon atoms. Write down its molecular formulae as
alkyne
View SolutionIf a saturated hydrocarbon has 4 carbon atoms, then the formula of the hydrocarbon could be _________.
View SolutionAn organic compounds has molecular formula C6H10O2. Which of the following class of compounds cannot have this molecular formula?
View Solution
