Find the efficiency of a cycle consisting of two isobaric and two isothermal lines if the pressure varies n−fold and the absolute temperature τ−fold within the cycle. The working substance is an ideal gas with the adiabatic exponent γ.
Here Q1''=CpT0(τ−1),Q'1''=τRT01nn and
Q2''=CpT0(τ−1),Q2'''=RT01nn
in addition to we have
Q1=Q1'+Q1'' and
Q2'=Q2''+Q2'''
So η=1−Q'2Q1=1−Cp(τ−1)+R1nnCp(τ−1)+τR1nn
=1−τ−1+(1−1γ)1nnτ−1+(1−1γ)τ1nn
=1−τ−1+(1−1γ)1nnτ−1+(1−1γ)τ1nn=(τ−1)1nnτ1nn+γ(τ−1)γ−1.
 .
.
Similar Questions
An ideal gas with the adiabatic exponent gamma goes through a cycle (Fig. 2.3) within which the absolute temperature varies tau-fold . Find the efficiency of this cycle. .
View SolutionOne mole of an ideal gas with the adiabatic exponent γ goes through a polytropic process as a result of which the absolute temperature of the gas increases τ−fold. The polytropic constant equal n. Find the entropy increment of the gas in this process.
View SolutionA working substance goes through a cycle within which the absolute temperature varies n-fold , and the shape of the cycle is shown in (a) Fig.a , (b) Fig. b, where T is the absolute temperature, and S the entropy. Find the efficiency of each cycle. .
View SolutionAn ideal gas goes through a cycle consisting of ischoric adiabatic and isothermal lines. The isothermal process is perform at minimum temperature. If the absolute temperature varies K times with tn the cycle then find out its effcincy.
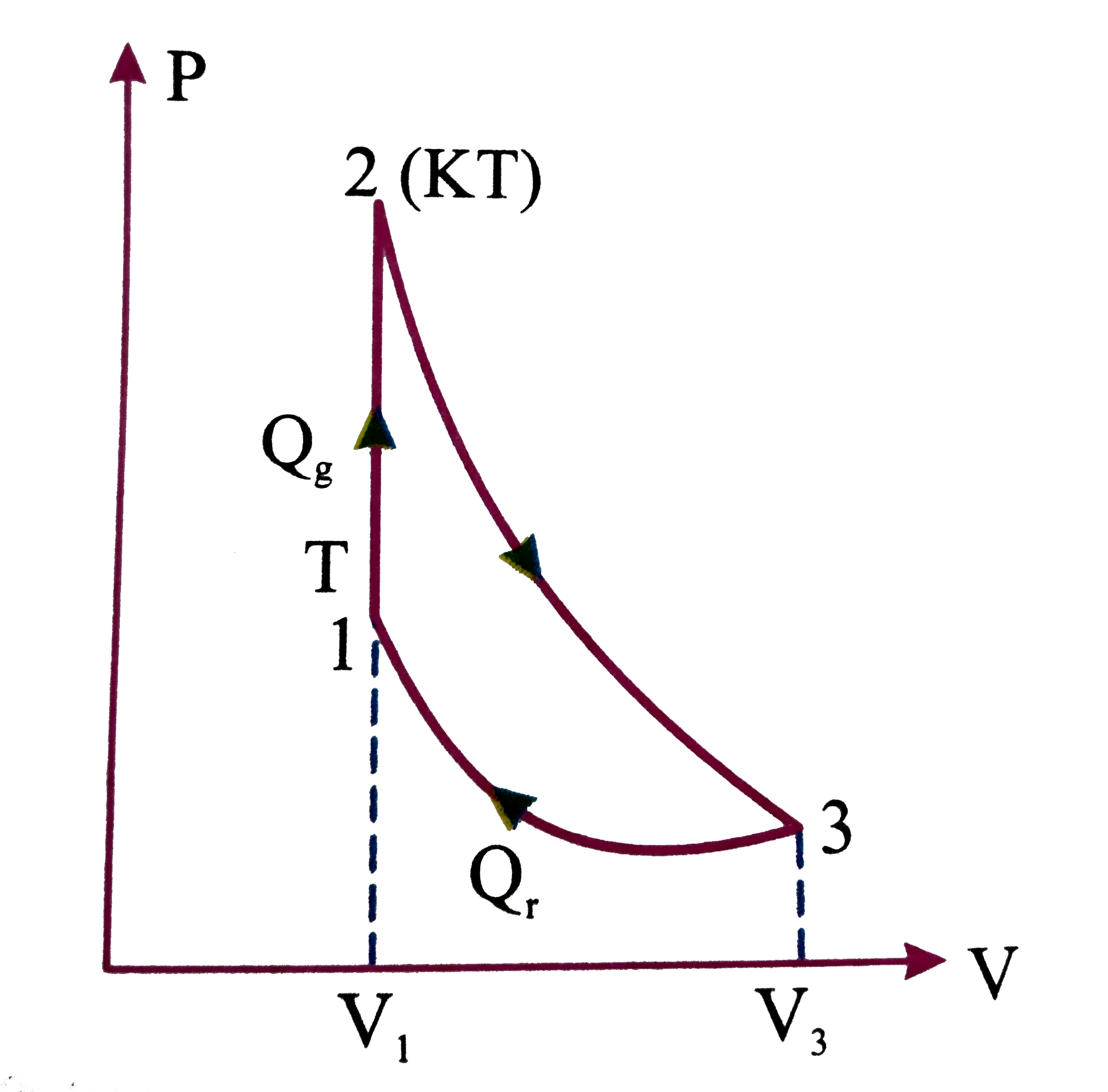
View SolutionOne mole of an ideal gas is carried through a thermodynamics cycle as shown in the figure. The cycle consists of an isochoric, an isothermal and an adiabatic process. Find the adiabatic exponent of the gas.
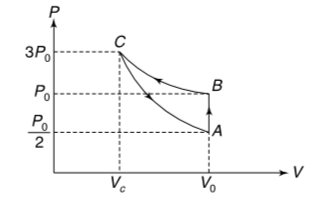
View SolutionAn ideal gas with a known gamma , completes a cycle consisting of two isotherms and two isobars. The isothermal processes are executed at temperatures T and T' (lt T) and isobaric processes are completed at pressures P_(0) and eP_(0) [e = base of natural logarithm]. Find the efficiency of the cycle.
View SolutionFind the efficiency of a cycle consisting of two isochoric and two adiabatic lines if the volume of the ideal gas changes n=10 times within the cycle. The working gas has gamma=1.5 .
View Solution
