इस अभिक्रिया के लिए 800 K पर Kc=4.24 है - CO(g)+H2O⇔CO2(g)+H2(g) 800 K पर CO2 एवं H2,CO तथा H2O के साम्य पर सांद्रताओं की गणना कीजिए , यदि प्रारंभ के केवल CO तथा H2O ही उपस्थित हो तथा प्रत्येक की सांद्रता 0 .1 M हो ।
निम्नलिखित अभिक्रिया के लिए ,
CO(g)+H2O⇔CO2(g)+H2(g)
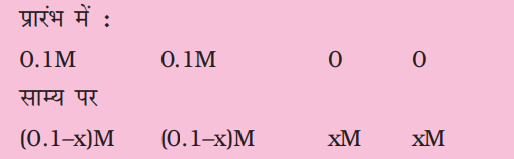
जहां साम्य पर CO2 तथा H2 की मात्रा xmolL−1 हैं
अतः साम्य स्थिरांक को इस प्रकार लिखा जा सकता हैं -
Kc=x2(0.1−x)2
=4.24
x2=4.24(0.01+x2−0.2x)
x2=0.0424+4.24x2−0.848x
3.24x2−0.848x+0.0424=0
a=3.24,b=−0.848,c=0.0424
एक द्विघात समीकरण के लिए ax2+bx+c=0,
x=−b±√b2−4ac2a
x=0.848
±√(0.848)2−4(3.24)(0.0424)/(3.24×2)
x=(0.848±0.4118)/6.48
x1=(0.848−0.4118)/6.48=0.067
x2=(0.848+0.4118)/6.48=0.194
मान 0.194 की उपेक्षा की जा सकती हैं ,क्योंकि यह अभिकारकों की सांद्रता बतलाएगा ,जो प्रारम्भिक सांद्रता से अधिक हैं |
अतः साम्यावस्था पर सांद्रताएँ यह हैं ,
[CO2]=[H2]=x=0.067M
[CO]=[H2O]=0.1−0.067=0.033M
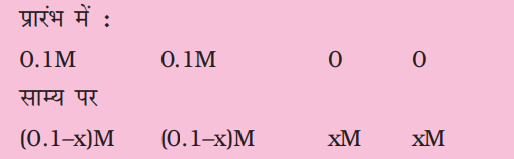
समान प्रश्न
The reaction was started with 0.1 M each of CO and H_(2)O at 800K. K_(C) for the reaction, CO(g)+H_(2)O (g) Leftrightarrow CO_(2) (g)+H_(2) (g) at 800K, 4.24. What is the equilibrium concentration of CO_(2) gas?
View SolutionCalculate delta H_(r)^(0) for the reaction CO_(2)(g) + H_(2)(g) rarr CO(g) + H_(2)O(g) given that Delta H_(r)^(0) for CO_(2) (g), CO (g) and H_(2)O (g) are - 393.5, -111.31 and - 242 KJ mol^(-1) respectively.
View SolutionThe value of Kc=4.24 at 800K for the reaction, CO(g)+H2O(g)⇔CO2(g)+H2(g)
Calcualte equilibrium concentrations of CO2, H2, CO and H2O at 800K, if only CO and H2O are present initially at concentrations of 0.1M each.
View SolutionThe value of Kc=4.24 at 800K for the reaction.
CO(g)+H2O(g)⇔CO2(g)+H2(g)
Calculate equilibrium concentration of CO2,H2,CO and H2O at 800K. If only CO and H2O are present initially at concentrations of 0.10M each.
View SolutionThe value of Kc=4.24 at 800 K for the reaction
CO(g)+H2O(g)⇔CO2(g)+H2(g)
Calculate equlibrium concentrations of CO2,H2,COandH2O at 800 K, if only CO and H2O are present initially at concentration of 0.10 M each?
View SolutionThe value of Kc=4.24 at 800K for the reaction, CO(g)+H2O(g)⇔CO2(g)+H2(g)
Calcualte equilibrium concentrations of CO2, H2, CO and H2O at 800K, if only CO and H2O are present initially at concentrations of 0.1M each.
View SolutionFor the equilibrium H2(g)+CO2(g)⇔⇔H2O(g)+CO(g),Kc=16 at 1000 K. If 1.0 mole of CO2 and 1.0 mole of H2 are taken in a l L flask, the final equilibrium concentration of CO at 1000 K will be
View Solution1260 K पर अभिक्रिया H2O(g)+CO(g)↔H2(g)+CO2(g) के लिए साम्य स्थिरांक 0.44 है। 1260 K पर अभिक्रिया 2H2(g)+2CO2(g)↔2CO(g)+2H2O(g) के लिए साम्य स्थिरांक कितना होगा?
View Solution

