Among the following, L− serine is
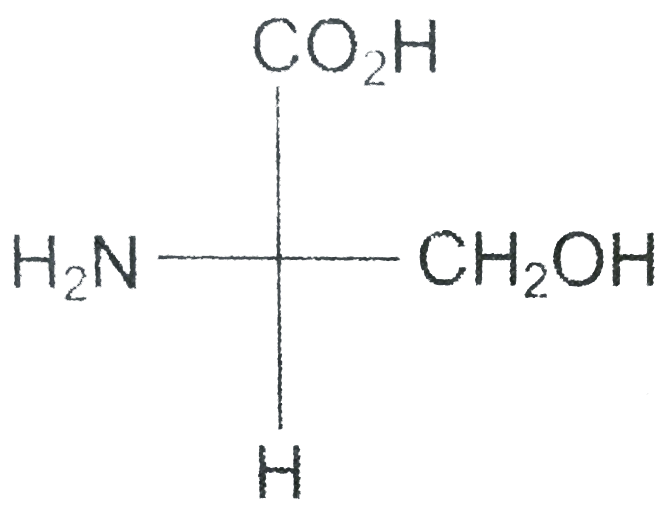
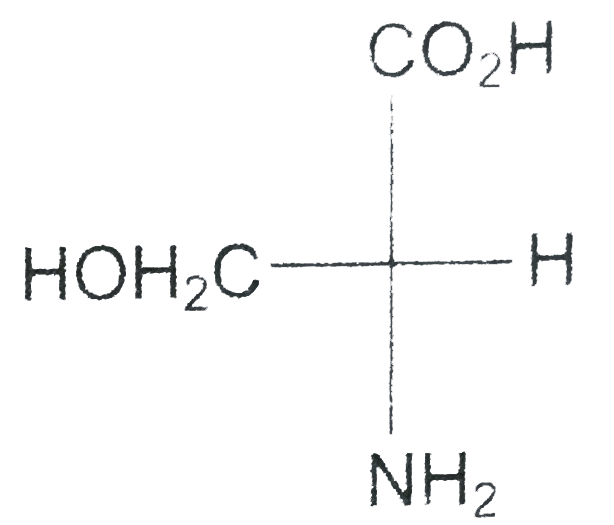
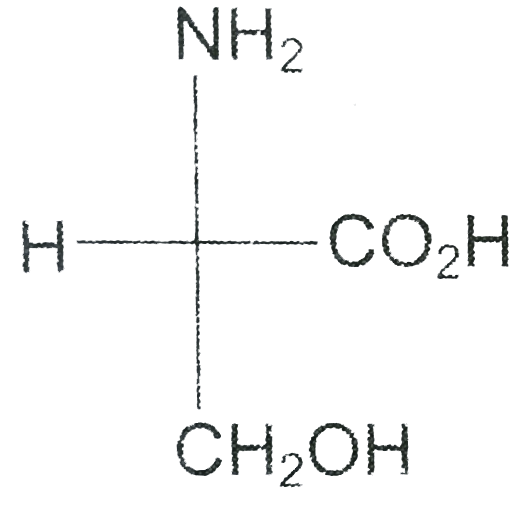
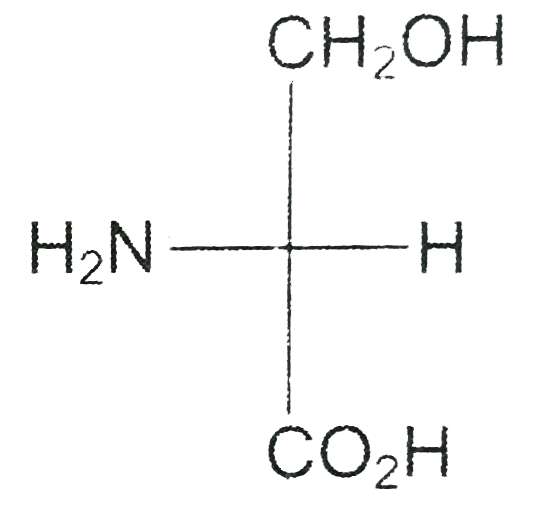
The correct Answer is:C
To determine which structure represents L-serine among the given options, we will follow these steps:
1. Understand the Structure of Amino Acids:
Amino acids consist of a central carbon atom (Cα) bonded to four different groups: an amino group (NH2), a carboxylic acid group (COOH), a hydrogen atom (H), and a variable side chain (R group). In the case of serine, the side chain is a hydroxymethyl group (CH2OH).
2. Identify the Configuration:
The designation of L or D for amino acids is based on the spatial arrangement of the groups around the central carbon atom. The L-form corresponds to a specific stereochemistry, which can be determined using the Cahn-Ingold-Prelog (CIP) priority rules.
3. Assign Priorities to the Groups:
According to the CIP rules, we assign priorities to the four groups attached to the central carbon based on atomic number:
- NH2 (amino group) has the highest priority (1).
- CH2OH (hydroxymethyl group) comes next (2).
- COOH (carboxylic acid group) is third (3).
- H (hydrogen) has the lowest priority (4).
4. Determine the Orientation of the Lowest Priority Group:
To determine whether the configuration is L or D, we need to look at the orientation of the lowest priority group (H) in relation to the other three groups. If the lowest priority group is oriented away from us (in the plane), we can analyze the arrangement of the other three groups.
5. Analyze the Arrangement:
- If the arrangement of the groups (1, 2, 3) is clockwise when viewed from the perspective of the lowest priority group (H) being away, it indicates a D configuration.
- If the arrangement is counterclockwise, it indicates an L configuration.
6. Conclusion:
After analyzing the structures provided, we find that the one where the arrangement of the groups (NH2, CH2OH, COOH) is counterclockwise with H oriented away from us represents L-serine.
Final Answer:
The structure that represents L-serine is the one with the counterclockwise arrangement of the groups when the hydrogen atom is oriented away.
---
Amino acids consist of a central carbon atom (Cα) bonded to four different groups: an amino group (NH2), a carboxylic acid group (COOH), a hydrogen atom (H), and a variable side chain (R group). In the case of serine, the side chain is a hydroxymethyl group (CH2OH).
The designation of L or D for amino acids is based on the spatial arrangement of the groups around the central carbon atom. The L-form corresponds to a specific stereochemistry, which can be determined using the Cahn-Ingold-Prelog (CIP) priority rules.
According to the CIP rules, we assign priorities to the four groups attached to the central carbon based on atomic number:
- NH2 (amino group) has the highest priority (1).
- CH2OH (hydroxymethyl group) comes next (2).
- COOH (carboxylic acid group) is third (3).
- H (hydrogen) has the lowest priority (4).
To determine whether the configuration is L or D, we need to look at the orientation of the lowest priority group (H) in relation to the other three groups. If the lowest priority group is oriented away from us (in the plane), we can analyze the arrangement of the other three groups.
- If the arrangement of the groups (1, 2, 3) is clockwise when viewed from the perspective of the lowest priority group (H) being away, it indicates a D configuration.
- If the arrangement is counterclockwise, it indicates an L configuration.
After analyzing the structures provided, we find that the one where the arrangement of the groups (NH2, CH2OH, COOH) is counterclockwise with H oriented away from us represents L-serine.
The structure that represents L-serine is the one with the counterclockwise arrangement of the groups when the hydrogen atom is oriented away.
Similar Questions
Knowledge Check
Among the following, the incorrect statement is:
ACellulose and amylose have 1,4-glycosidic linkageBLactose contains β-D-galactose and β-D-glucoseCMaltose and lactose have 1,4-glycosidic linkageDSucrose and amylose have 1,2-glycosidic linkageAmong the following, the incorrect statement is :
AAt low pressure, real gases show ideal behaviour.BAt very low temperature, real gases show ideal behaviourCAt Boyle's temperature, real gases show idela behaviourDAt very large volume, real gases show ideal behaviour.Among the following strongest base is
ALa(OH)3BCe(OH)3CLu(OH)3DYb(OH)3
