A simple mechanism for enzyme-catalyzed reaction is given by the folllwoing set of equations
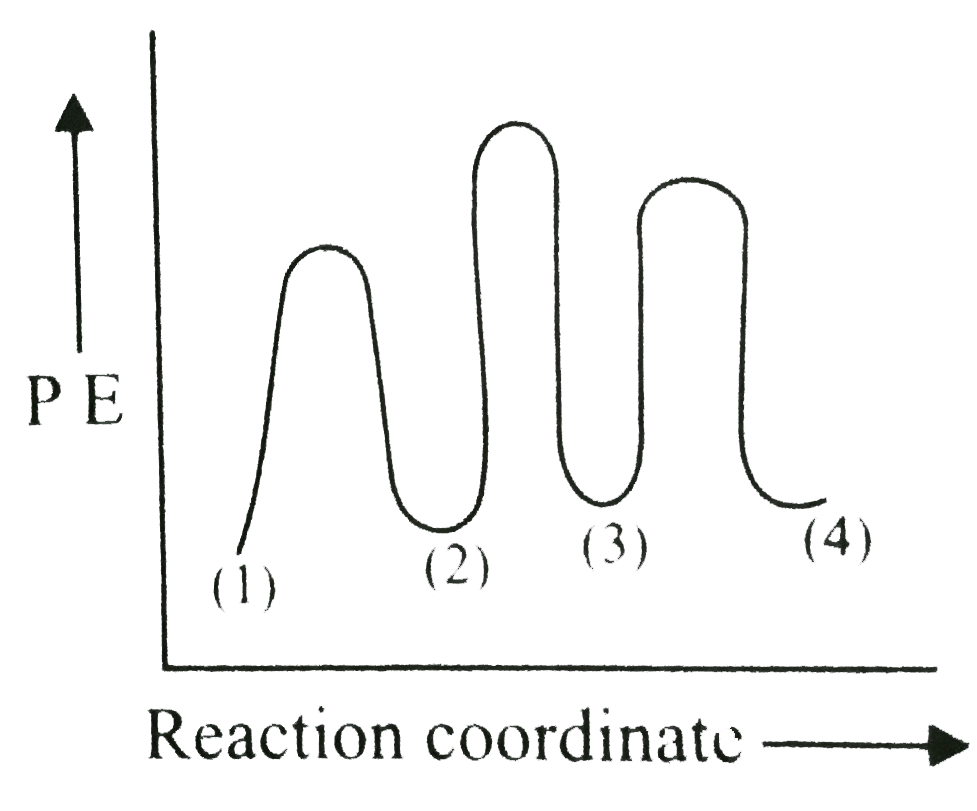
E(enzyme)+S(reactant)⇐⇒ES(intermediata 1)ES(intermediate 1)⇐⇒EP(intermediate 2)EP(intermediate 2)⇐⇒E(enzyme)+P(product)
This is know as the Michaelis-Menten mechanism. The potential energy diagram is shown in the fig. Which of the following sets of identifications is correct ? (Assume that the temperature and pressure are constant).
(1)=E+P.,(2)=EP,(3)=ES,(4)=E+S
(1)ES, (2)Activatedcomplex, (3)EP, (4)Activatedcomplex
(1)EP (2)Activatedcomplex, (3)ES, (4)Activatedcomplex
(1)E+S (2)ES (3)EP (4)E+P
The correct Answer is:D
Intermediate and product have less potential energy than activated complex.
Similar Questions
Predict the correct intermediate and product in the following reaction : H_3C-C-=CH underset(HgSO_4)overset(H_2O, H_2SO_4)to underset"(A)""Intermediate" to underset"(B)""Product"
View SolutionWhich of the following names are not correct for the given compound?
CH2∣CHO−CH∣CHO−CH2∣CHO
View SolutionPredict the correct intermediate and product in the following reaction :
H3C−C≡CHH2O,H2SO4−−−−−−−−→HgSO4intermediate(A)→product(B)
View SolutionPredict the correct intermediate and product in the following reaction :
H3C−C≡CHH2O,H2SO4−−−−−−−→HgSO4 underset(( B)) underset("Product ") underset(darr ) underset((A)) ontermediate
View Solution¯¯¯CH2−C∣∣O..:−CH3↔CH2=CH2C∣:O:..−−CH3
View SolutionExplain how the hybrid structure is related to the structures of each of the following pairs of contributing resonance structures?
(a) ⊕CH2−(i)ΘC..H2↔CH2=(ii)CH2
(b) H2C=(iii)..O:↔H2C−(iv)..O..:−
(c ) R2+C−C∣R(v)=..O:↔R2C=C∣R−..O:+(vi)
(d) R−C+=..O:(VII)↔R−C≡O:+(VIII)
(e ) R−C∣∣:O:−..O..H(IX)↔R−C∣:O..:−⊕O..H(IX)
View SolutionR−C∣∣O−NH2 तथा Br2और KOH की अभिक्रिया में मध्यवर्ती उत्पाद प्राप्त होते हैं
View SolutionCompare the stabilities of the following intermediates :
H−+C∣H−H(a)
H−+C∣H−H(b)
View Solution

