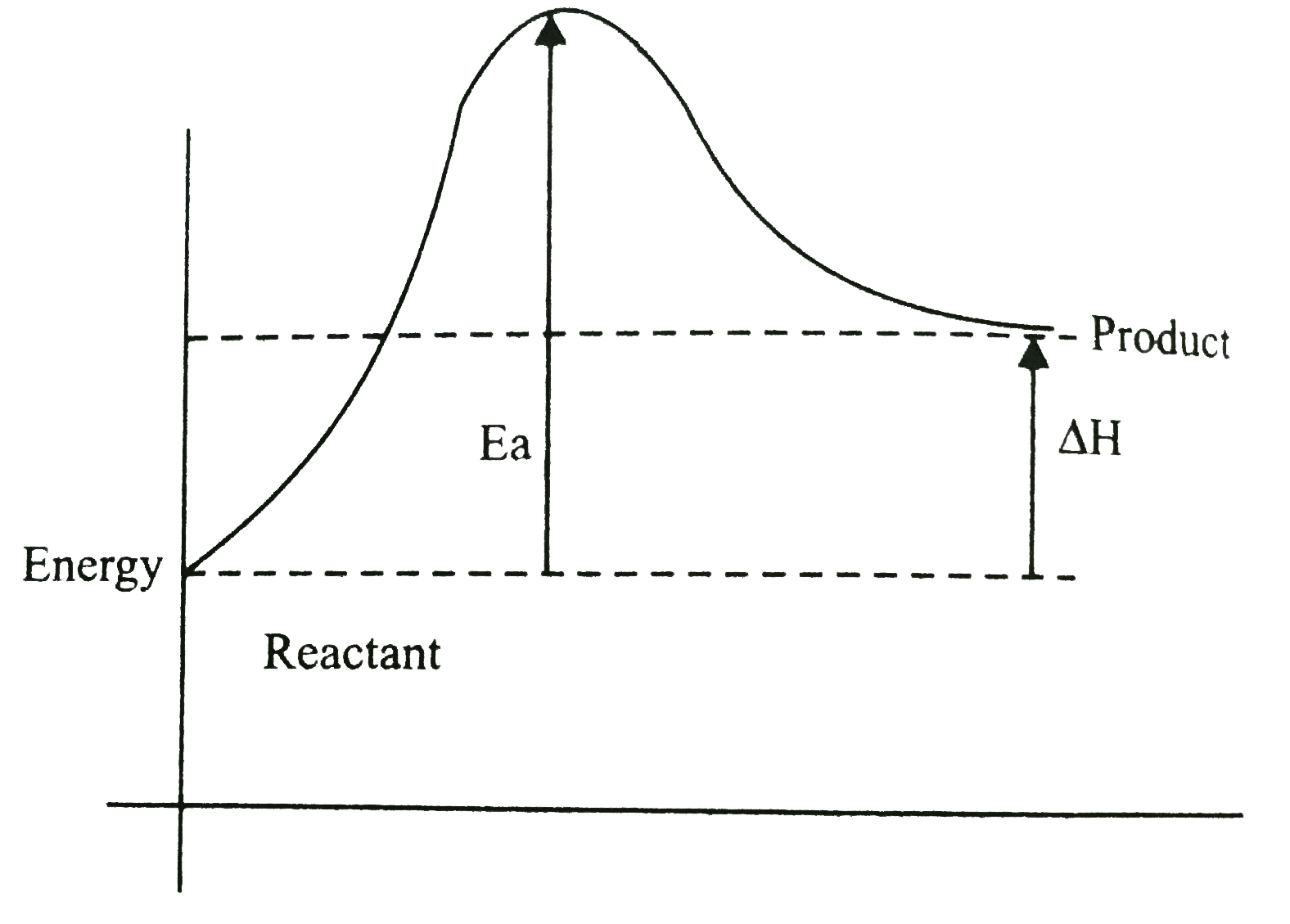
For an endothermic reaction energy of activation is Ea and enthalpy of reaction is ΔH (both in kJmol−1). Minimum value of Ea will be
<ΔH
=ΔH
>ΔH
=0
The correct Answer is:C
Similar Questions
The reaction X→Y is an exothermic reaction. Activation energy of the reaction for X into Y is 150 kJ mol−1. Enthalpy of reaction is 135 kJ mol−1. Calculate the activation energy for the reverse reaction, Y→X in kJmol−1.
View SolutionFor an endothermic reaction, energy of activation is Ea and enthalpy of reaction is ΔH (both of these in kJ/mol). Minimum value of E will be
View Solution
Knowledge Check
For an endothermic reaction energy of activation is Ea and enthalpy of reaction is ΔH (both in kJmol−1). Minimum value of Ea will be
A<ΔHB=ΔHC>ΔHD=0For an endothermic reaction, energy of activation is Ea and enthalpy of reaction is ΔH (both of these in kJ/mol). Minimum value of E will be
Aless than ΔHBequal to ΔHCmore than ΔHDequal to zeroFor an endothermic reaction, energy of activation is Ea and enthalpy of reaction is ΔH (both of these in kJ/mol). Minimum value of E will be
AEqual to zeroBLess than ΔHCEqual to ΔHDMore than ΔH
For an endothermic reaction, where ΔH represents the enthalpy of reaction in kJmol−1, the minimum value for the energy of activation will be
View SolutionFor an endothermic reaction, where ΔH represents the enthalpy of reaction in kJmol−1, the minimum value for the energy of activation will be
View SolutionFor an endothermic reaction, where ΔH represents the enthalpy of reaction in kJmol−1, the minimum value for the energy of activation will be
View SolutionFor an endothermic reaction, ΔH represents the enthalpy of the reaction in kJmol−1. The minimum of activation energy will be:
View SolutionFor an exothermic reaction an activation energy of 70 KJ mole −1 and the enthalpy change of reaction is 30 KJ mole −1 . The order of the reaction is
View Solution