Which has the stongest bond?
A
F−F
B
F−Cl
C
F−Br
D
Cl−B
Video Solution
Text Solution
The correct Answer is:A
F−F more strong bond compare to F−Cl,F−Br and Cl−Br bond.
|
Similar Questions
Given below is the graph representing the ionsiation energies of a few element ,A to H with successive atomic number
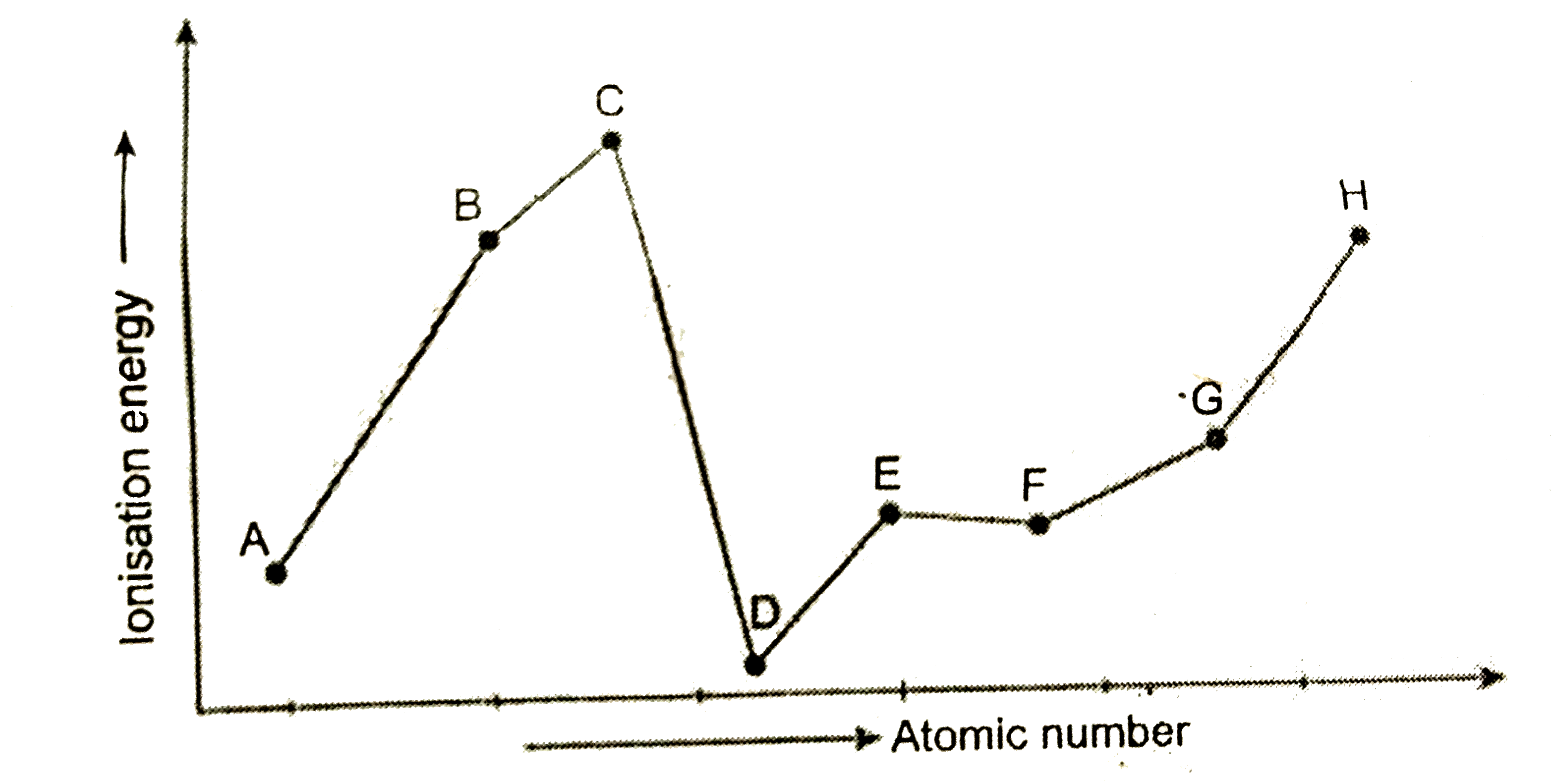
(a) identify the noble gas
(b) which of the following elements could Dbe?
(c ) which element has the highest electron affinity
(d) Which element has the stongest metallic character?
(e ) write the unit of ionisation energy
View SolutionWhich has the largest bond dissociation energy?
View Solution
Knowledge Check
Which has the strongest bond?
AI2>ICl>HI<HIO4BHIO4<ICl<I2<HICI2<HI<ICl<HIO4DHi<I2<ICl<HIO4Which has the highest bond energy?
AF2BCl2CBr2DI2Which of the following has the strongest H - bond?
AO−H−−−OBS-H---SCF−H−−−FDN−H−−−N

