Which of the following reaction is/are possible?
A
K[BrICl]Δ−−→KCl+Ibr
B
K[BrICl]Δ−−→KBr+Icl
C
K[BrICl]Δ−−→KI+BrCl
D
All of the above\
Video Solution
Text Solution
The correct Answer is:A
K[BrICl]Δ−−→KCl+Ibr
|
Similar Questions
Knowledge Check
Which of the following reactions is / are possible at the anode ?
AF2+2e−→2Fc−B2H⊕+12O(2)+2e−→H2OC.2 C r 3 + + 7 H 2 O → C r 2 O 2 − 7 + 14 H ⊕ + 6 e −DFe2+→Fe3++e−Which of the following reactions is not possible ?
A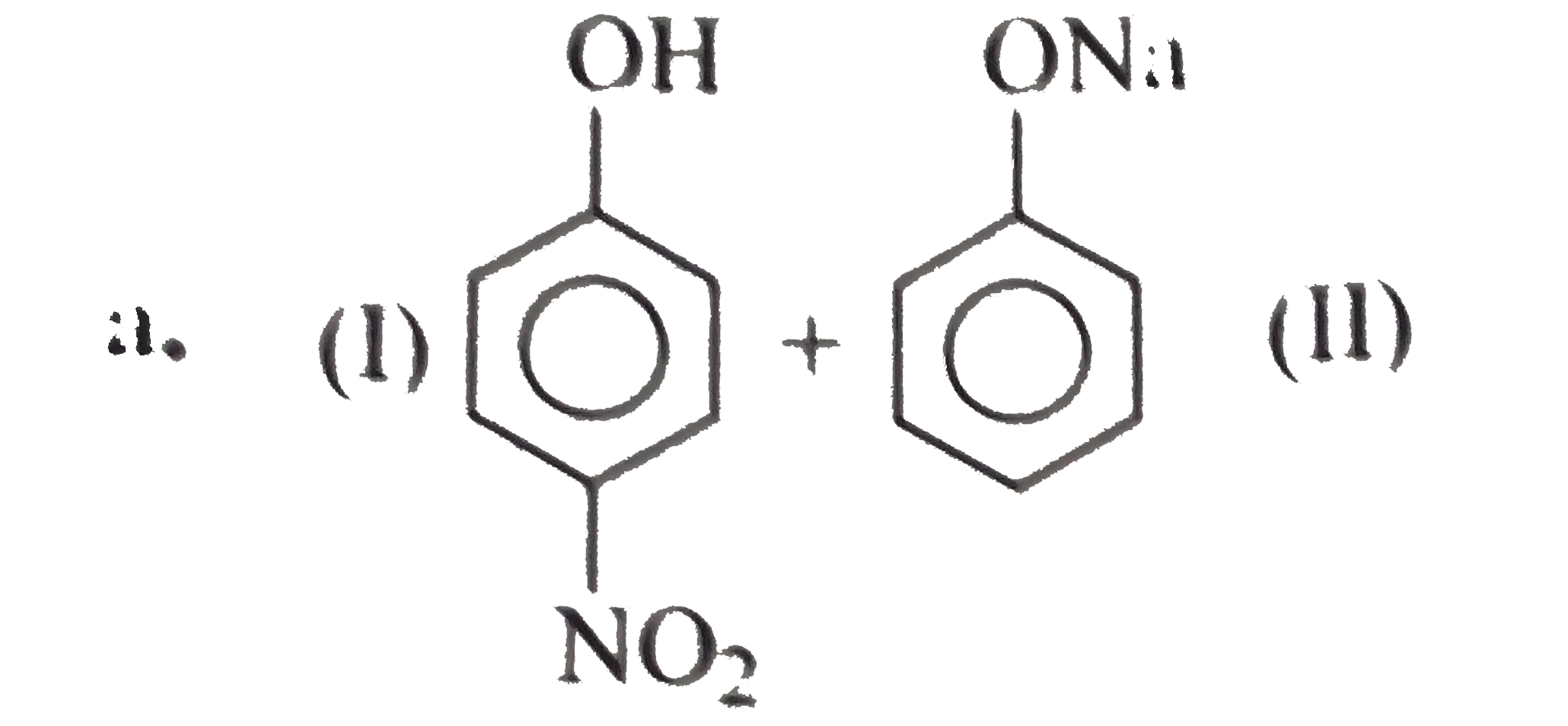 B
B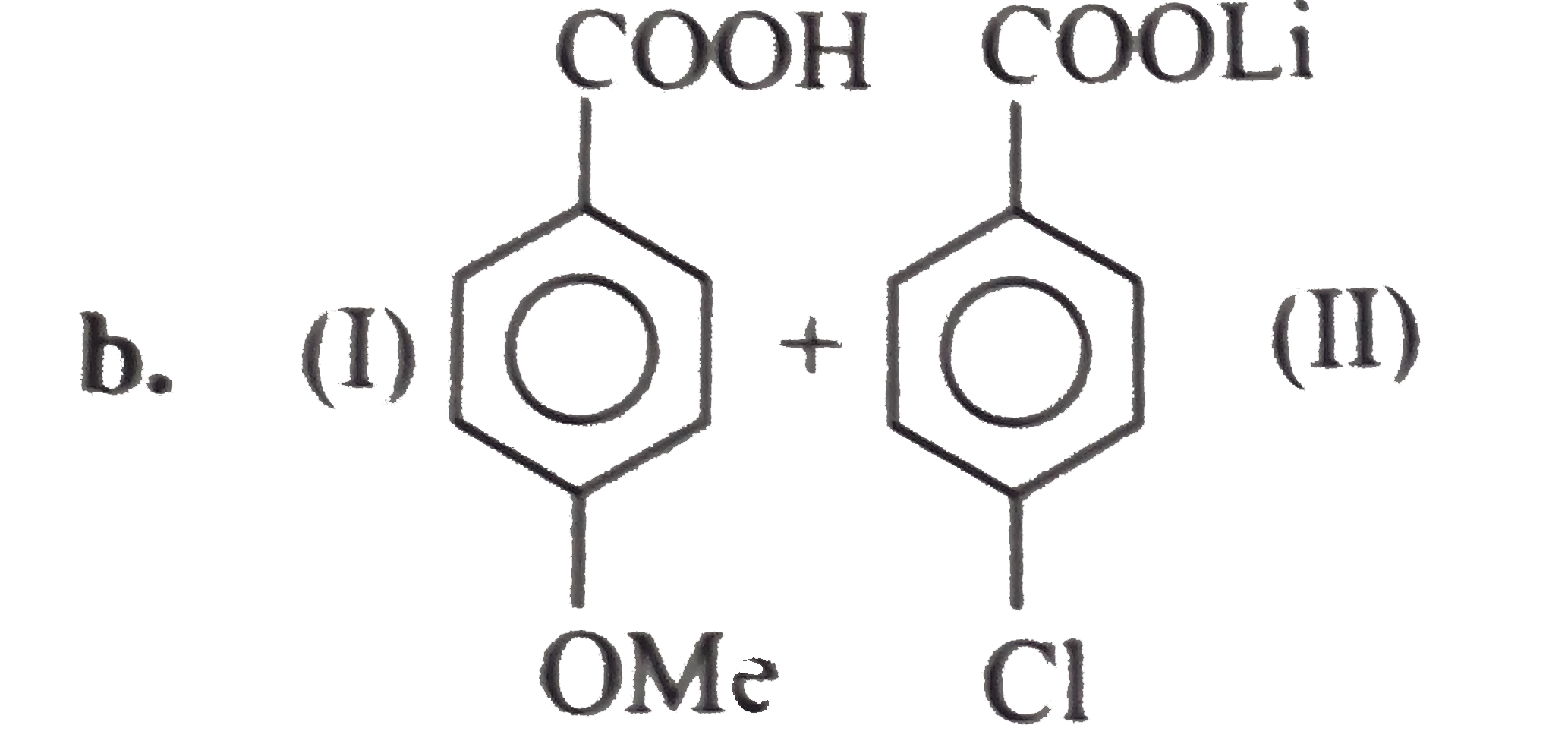 C(I)CH3OH+C2H5Ona(II)D(I)ClCH2COOH+CH3COONa(II)
C(I)CH3OH+C2H5Ona(II)D(I)ClCH2COOH+CH3COONa(II)Why the following reaction is not possible?
ABoth Cr2O2−7andFe3+ are reducing agentsBBoth Cr2O2−7andFe3+ are oxidising agentsCCr2O2−7 is a strong oxidsing agent while Fe3+ is a weak oxidising agent.DThe solution is acidic in nature

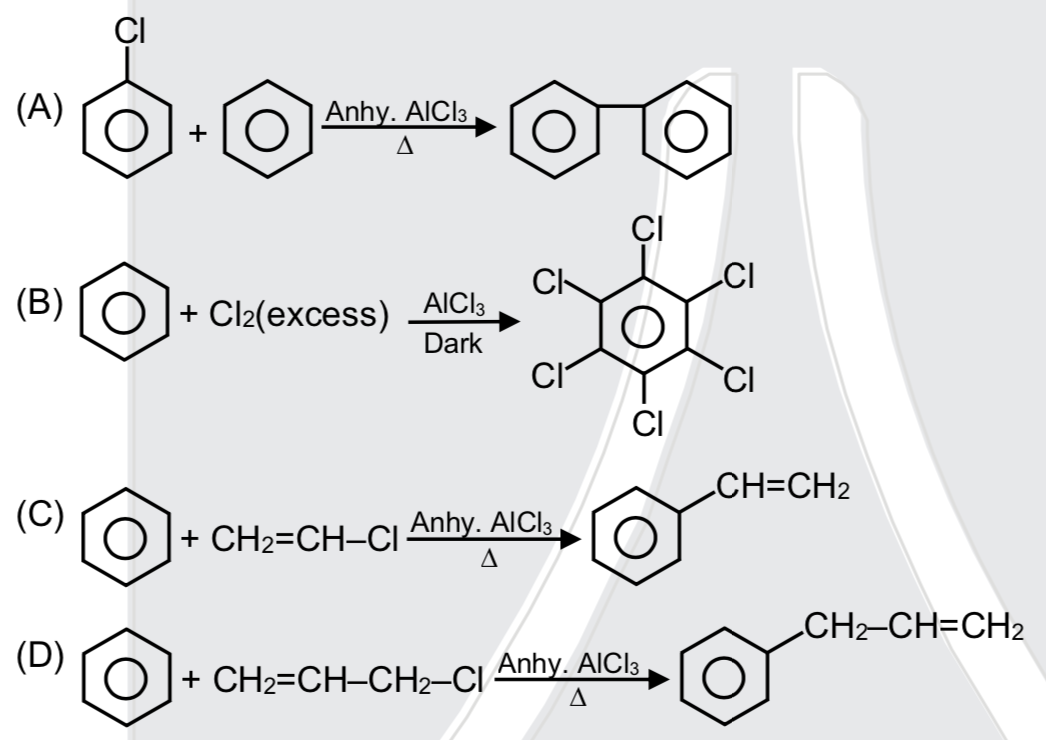
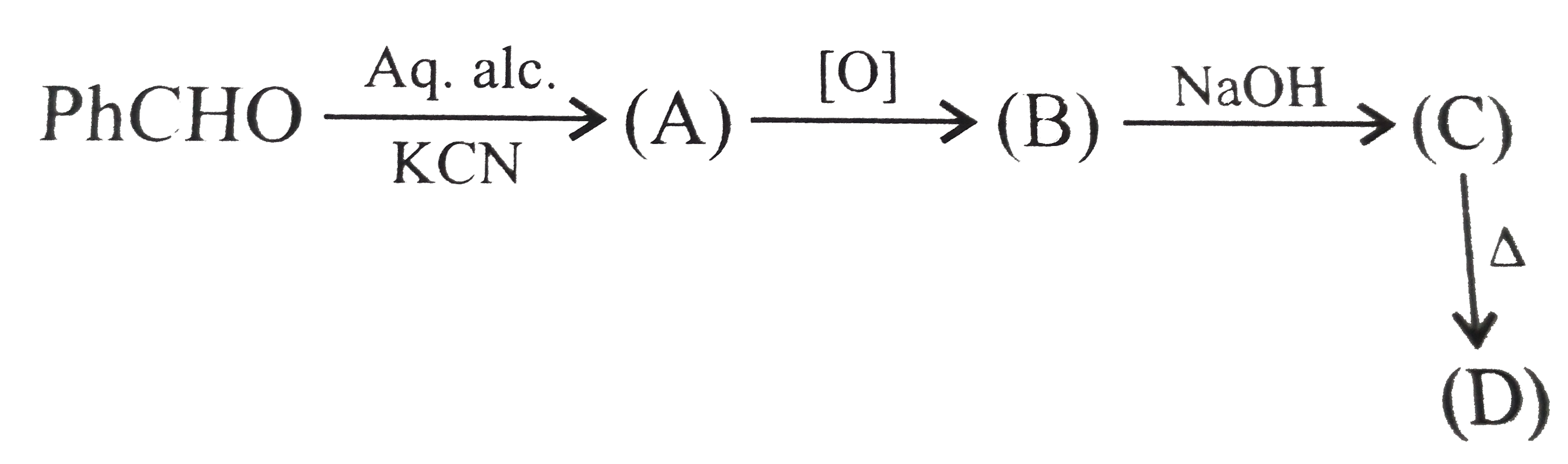
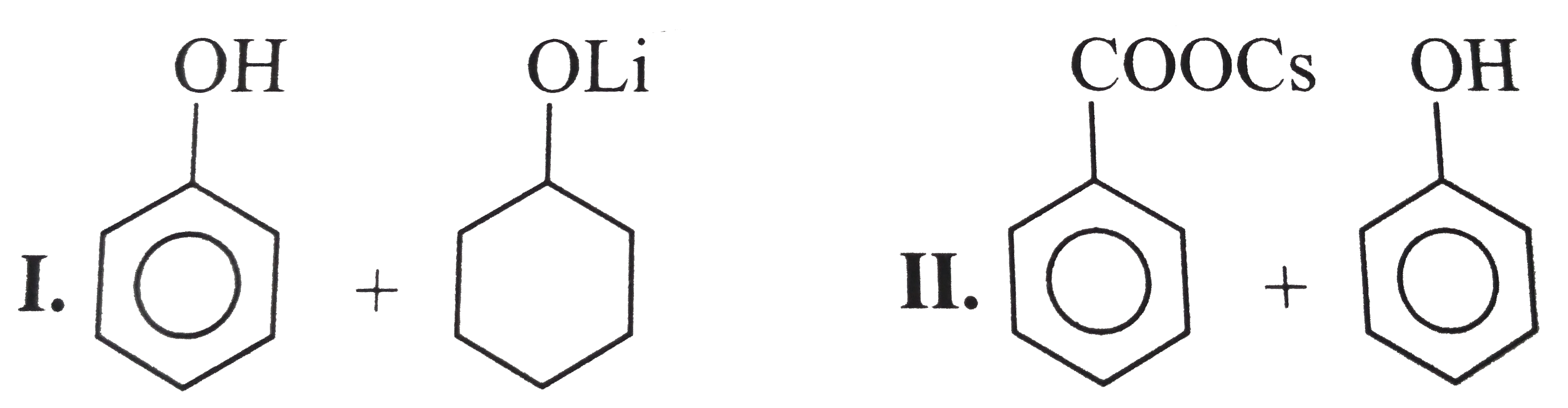
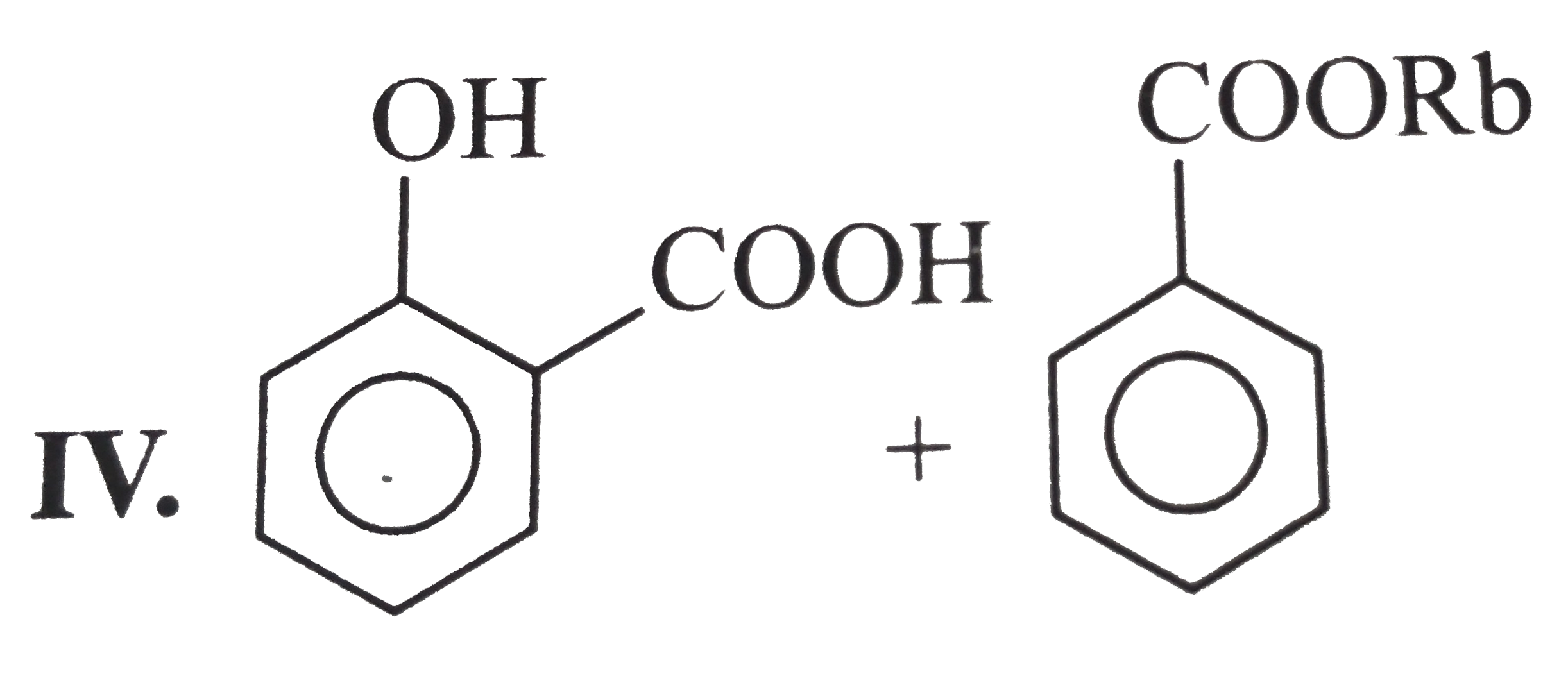 .
.